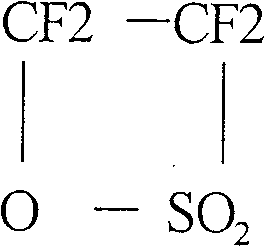
Process for preparing (per)fluorohaloethers
A technology of fluorinated and fluoropolyether, which is applied in the field of preparing fluorinated vinyl ethers with fluorosulfonic acid groups, can solve the problems of catalyst poisoning and activity reduction, and achieve high productivity, simplified methods, and equipment parts Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 (comparative example)
[0051] Synthesis of FSO according to prior art 2 -CF 2 -CF 2 O-CFCl-CF 2 Cl
[0052] Fluorine diluted with 1.5Nl / h nitrogen (fluorine / nitrogen molar ratio 1 / 14) and 1.1Nl / h SO 2 F-CF 2 - The COF is fed into a 500cc metal reactor filled with catalyst CsF mixed with copper wire to disperse the heat of reaction. Conversion of acyl fluoride to hypofluorite SO 2 F-CF 2 -CF 2 The yield of OF was 99.5%. The hypofluorite prepared in this way is diluted with nitrogen again (hypofluorite / nitrogen molar ratio 1 / 35), and then sent to a tank containing 69g CFCl=CFCl (CFC 1112) and 453g CF 2 Cl-CF 3 (CFC 115) In the CSTR type reactor (continuous stirred tank reactor) used as the reaction solvent, the temperature was kept at -85°C.
[0053] After 4 hours the autoclave was opened and the resulting solution was analyzed by gas chromatography.
[0054] The reaction mass balance was 97.1%. The conversion rate of hypofluorite is 100%, its ...
Embodiment 2
[0057] Synthetic FSO 2 -CF 2 -CF 2 O-CFCl-CF 2 Cl
[0058] 441g CF 2 Cl-CF 3 (CFC 115) was added to the CSTR reactor of Example 1, and the temperature was maintained at -80°C. Then feed 3.9Nl / h of nitrogen-diluted fluorine (fluorine / nitrogen molar ratio 1 / 5), 3.1Nl / h of CFCl=CFCl (CFC 1112) and 3.9Nl / h of SO 2 F-CF 2 -COF.
[0059] The reaction was carried out for 3 hours, then the autoclave was opened: the mass balance was 97.4%. The initial product of the reaction was distilled in a metal column, and the fractions obtained were analyzed by gas chromatography and 19 F NMR analysis. CFC 1112 was completely converted and the conversion of acid fluoride was 57.8%.
[0060] Isolated 52.7g fluorosulfonic acid adduct FSO 2 -CF 2 -CF 2 O-CFCl-CF 2 Cl. The selectivity was 49.8%.
[0061] 39.6 g of acid fluoride were recovered.
[0062] The main reaction also occurs the fluorination of CFC 1112 to CFC 114 and to CF 2 Cl-CFCl-CFCl-CF 2 Fluorinated dimerization of Cl...
Embodiment 3
[0065] Preparation of FSO by Excessive Acyl Fluoride 2 -CF 2 -CF 2 O-CFCl-CF 2 Cl
[0066] Repeat Example 2 under the same conditions, add 128.9g SO to the CSTR reactor 2 F-CF 2 -COF and 311.1g solvent (CFC 115), feed 3.9Nl / h of nitrogen-diluted fluorine (fluorine / nitrogen molar ratio 1 / 5), 3.1Nl / h of CFCl=CFCl (CFC 1112) and 3.9 SO of Nl / h 2 F-CF 2 -COF.
[0067] The reaction mass balance was 97.6%.
[0068] Similar to Example 2, the initial product of the reaction is distilled in a metal column, and the cut obtained is passed through gas chromatography and 19 F NMR analysis. CFC 1112 was completely converted, and the conversion of the charged acid fluoride was 29%.
[0069] Isolated 34.2 g of fluorosulfonic acid adduct FSO 2 -CF 2 -CF 2 O-CFCl-CF 2 Cl. Selectivity was 65.0%.
[0070] 39.6 g of acid fluoride were recovered.
[0071] In addition to the main reaction also occurs the fluorination of CFC 1112 to CFC 114 and to CF 2 Cl-CFCl-CFCl-CF 2 Fluorinate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com