An anti-tumor pharmaceutical composition
A technology of anti-tumor drugs and compositions, applied in drug combination, anti-tumor drugs, drug delivery and other directions, can solve problems such as toxic and side effects, and achieve the effects of reducing toxic and side effects, facilitating quality control, and improving drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The notoginseng medicinal material is crushed to the size of soybeans to coarse powder, extracted with 20%-80% water-containing alcohol under heat reflux, recovered solvent, dissolved in water or dilute alcohol, and eluted with alkaline water, 30%, 70% ethanol through D101 macroporous resin , reclaim 70% ethanol eluate solvent, obtain Panax notoginseng total saponins. Adopt high performance liquid phase method (HPLC) to detect it, analysis condition is Agilent Extend-C18, mobile phase H 2 O-CH 3 CN, the detection wavelength is 203nm, respectively with notoginseng saponin R 1 , Ginsenoside Rg 1 , Ginsenoside Rb 1 , Ginsenoside Rd, and Ginsenoside Re are the detection components, and their contents are determined by the external standard method. The contents of the five components are respectively notoginsenoside R 1 6%, Ginsenoside Rg 1 42%, Ginsenoside Rb 1 23%, ginsenoside Rd 7%, ginsenoside Re 3%.
[0014] Grind the medicinal materials of Sophora flavescens, ...
Embodiment 2
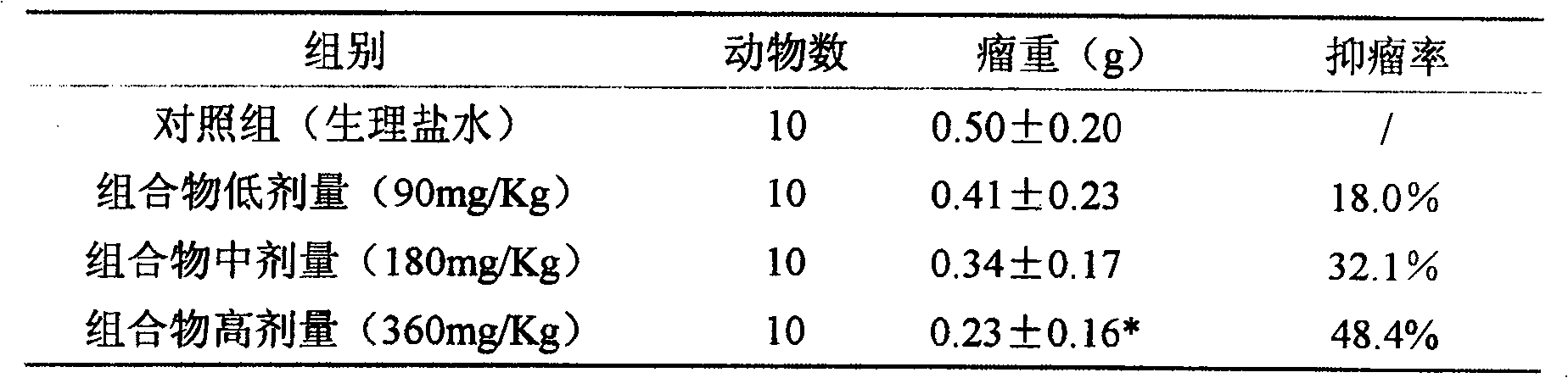
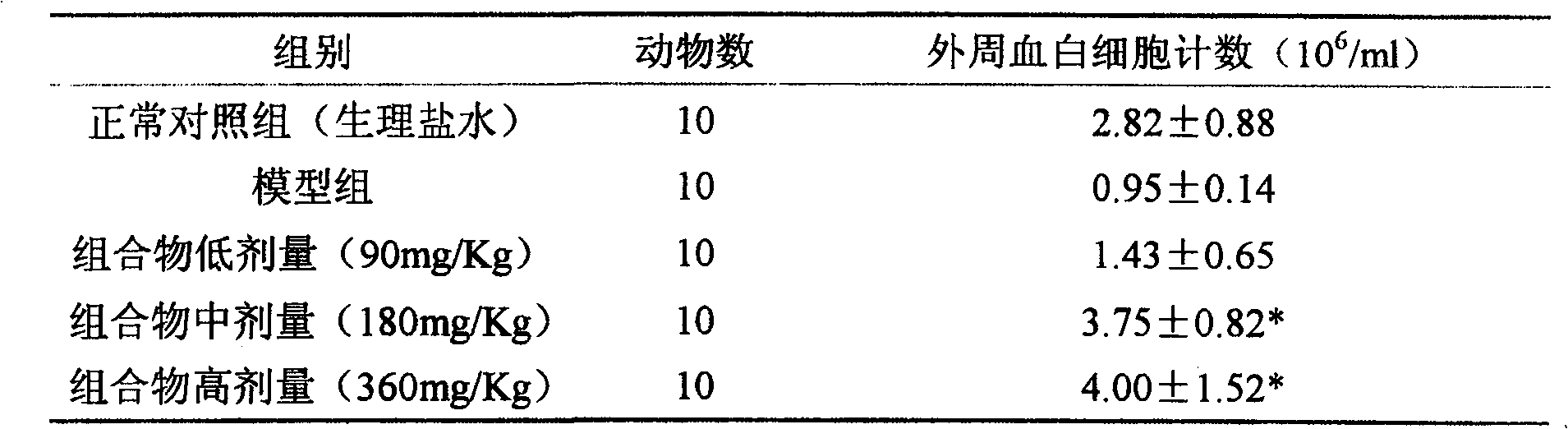
[0016] Antitumor effect experiment of composition 1 (total saponins of notoginseng:total alkaloids of matrine=1:1.5)
[0017] The mice pre-inoculated with H22 ascites tumor were sacrificed, and 1ml of ascites tumor was extracted from the abdominal cavity, and diluted with normal saline to 2×10 5 cells / ml, injected into the underarm of ICR female mice at 0.2ml / only, and after 24 hours, they were randomly divided into groups and started to administer. The compositions were administered in high, medium and low doses respectively. After 8 days of continuous administration, the drug was stopped, the mice were weighed, and the tumor pieces were dissected and weighed separately for statistical analysis. See Table 1 for the results.
[0018] The experimental therapeutic effect of table 1 composition on mouse H22 liver cancer
[0019]
[0020] Compared with the control group, * P<0.05
Embodiment 3
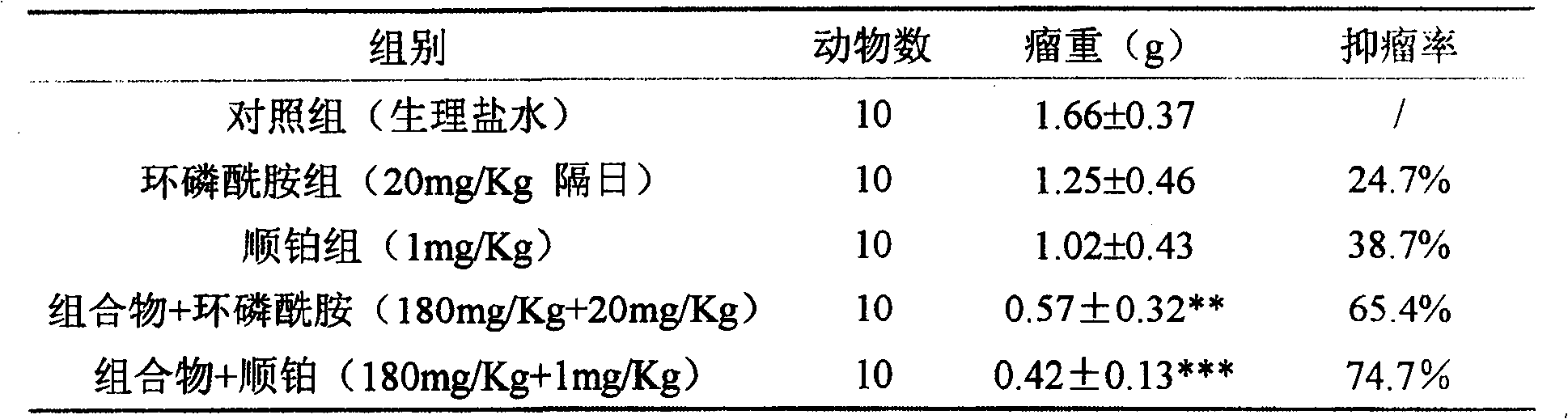
[0022] Synergistic experiment of composition (total saponins of Panax notoginseng:total alkaloids of matrine=2:1) and chemotherapeutic drugs
[0023] The mice pre-inoculated with H22 ascites tumor were sacrificed, and 1ml of ascites tumor was extracted from the abdominal cavity, and diluted with normal saline to 2×10 5 cells / ml, injected into the underarm of ICR female mice at 0.2ml / only, and after 24 hours, they were randomly divided into groups and started to administer. The dosage of the chemotherapeutic drug cyclophosphamide is 20 mg / Kg, intramuscular injection, and administered every other day; the dosage of cisplatin is 1 mg / Kg, intraperitoneal injection, and daily administration. The composition was intragastrically administered at a dose of 360 mg / Kg, once a day, and the results are shown in Table 2.
[0024] Table 2 Composition combined with chemotherapeutic drugs for experimental therapeutic effect on H22 liver cancer in mice
[0025]
[0026] Compared with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com