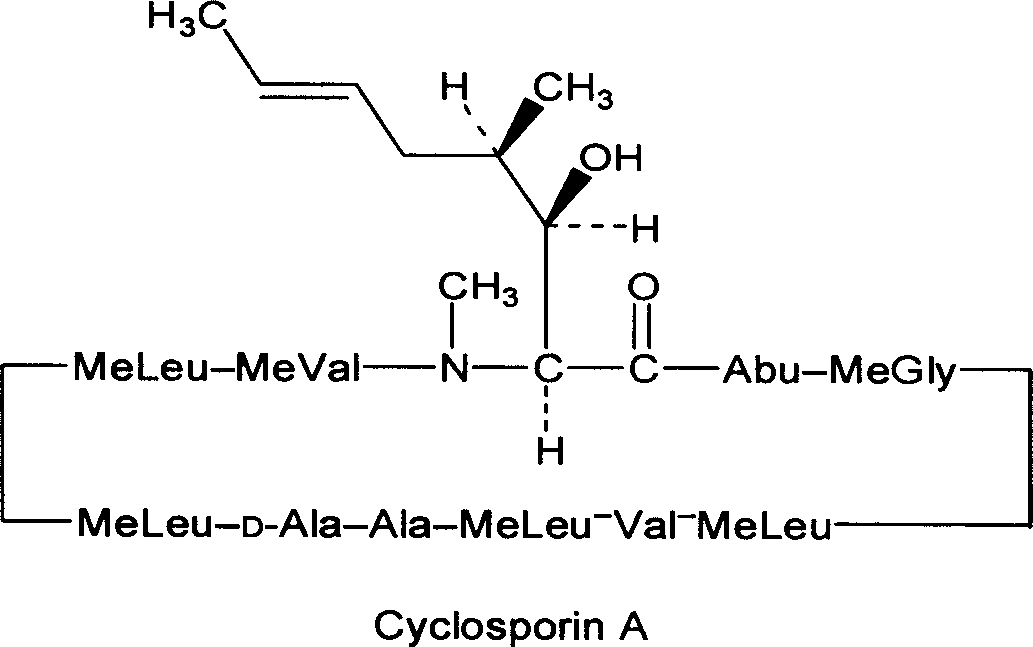
Cyclosporin A semisolid skeleton capsule and its preparation method
A cyclosporin and semi-solid technology, which is applied in the field of medicine, can solve problems such as increasing costs, increasing process steps, and accelerating the cross-linking and curing reaction of capsule shell gelatin, and achieves the effect of stable properties and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Mix 1 g of cyclosporine, 3 g of polyoxyethylene castor oil diglyceride, 2 g of ethyl oleate, and 4 g of poloxamer, stir evenly, heat and dissolve, and make 100 capsules with a capsule liquid filling machine, and the content of cyclosporine is 0.100g / grain.
Embodiment 2
[0025] Mix cyclosporine 1g, polyoxyethylene castor oil, polyoxyethylene 6 sorbitan monoglyceride 6g, maisine 3g, polyethylene glycol-4000 6g, stir evenly, heat to dissolve, and make it with a capsule liquid filling machine 100 capsules, the content of cyclosporine is 0.100g per capsule.
Embodiment 3
[0027] Mix 1 g of cyclosporine, 2 g of polyoxyethylene castor oil and Tween-80 mixture (1:1, weight ratio), 2 g of soybean oil, and 5 g of polyethylene glycol, stir evenly, heat to dissolve, and use a capsule liquid filling mechanism Into 100 capsules, the cyclosporine content is 0.100g / capsule.
[0028] In the above-mentioned embodiment, cyclosporin A, oil phase, surfactant with HLB>12 and co-emulsifier are added according to the aforementioned ratio, and the rest is a semi-solid skeleton carrier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com