Production method of m-bromophonol and production device thereof
A production method and m-bromophenol technology are applied in the production of m-bromophenol and the field of its production equipment, which can solve the problems of difficult operation, high equipment requirements, and low product yield, so as to avoid side reactions, shorten reaction time, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: a kind of production method of m-bromophenol, carries out following steps successively:
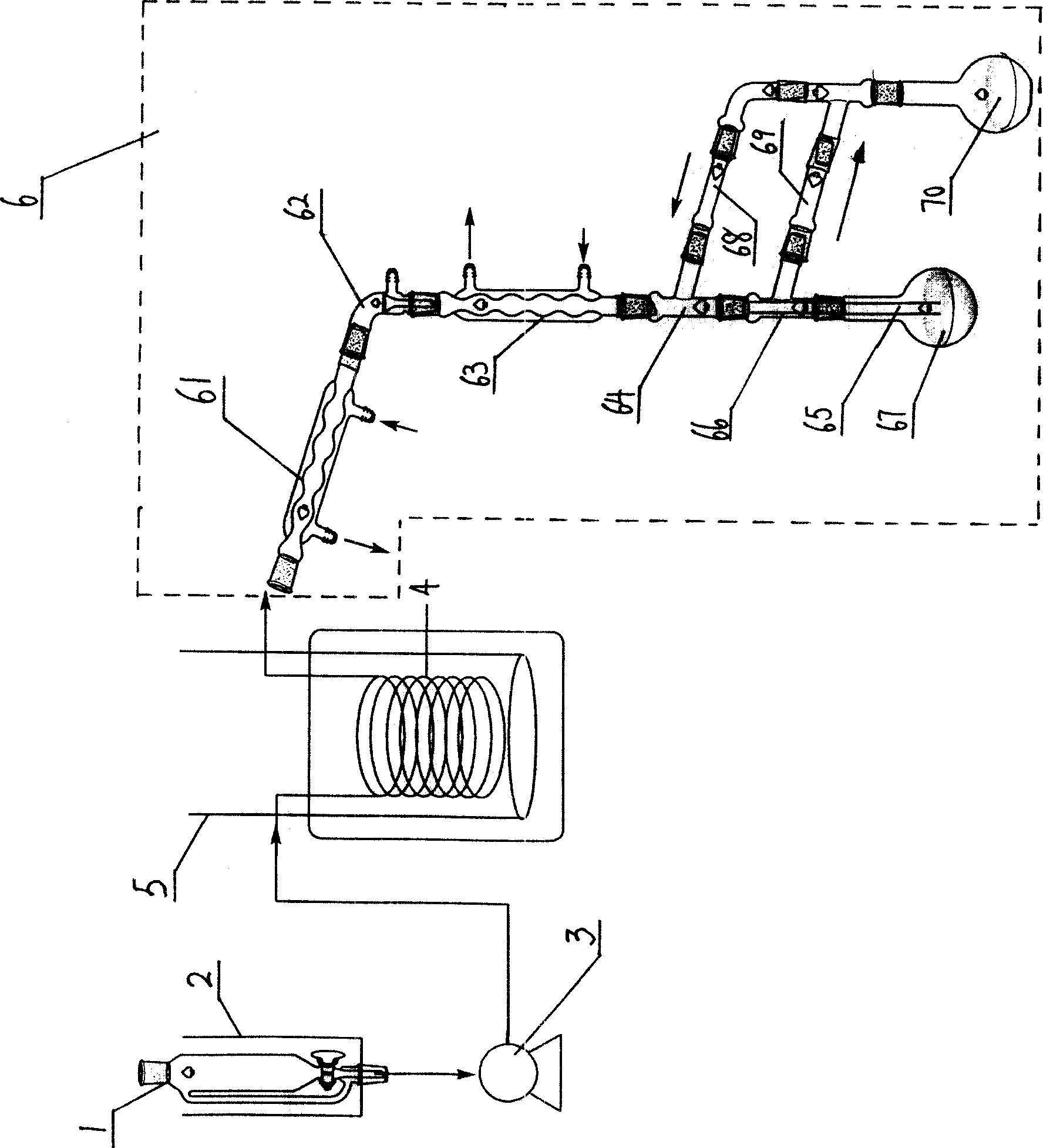
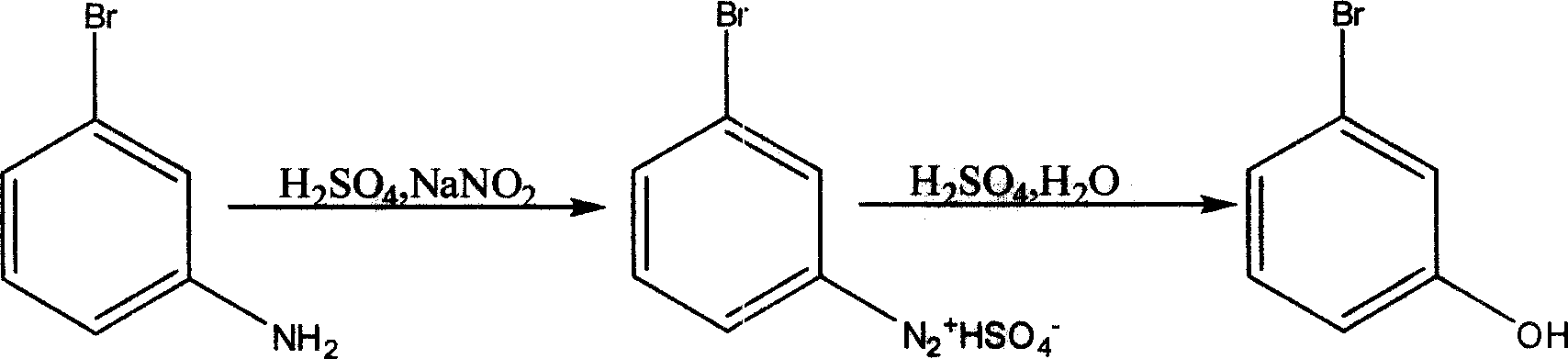
[0024] 1), diazotization reaction: a typical method is as follows (Preparation of m-bromophenol, C.FrederickKoelsch, Journal of the American Chemical Society (1939), 61, 969): 10g m-bromoaniline is dissolved in boiling 300ml water and 50ml sulfuric acid solution, the mixture was cooled to 10°C and diazotized with 4.0 g of sodium nitrite. 300 ml of the obtained m-bromoaniline diazosulfate aqueous solution (containing 0.0578 mol of m-bromoaniline diazosulfate).
[0025] 2), hydrolysis reaction: after the above-mentioned m-bromoaniline diazosulfate aqueous solution passes through the flow pump 3, it enters the pipeline reactor 4 with a flow rate of 100ml / hr to carry out the hydrolysis reaction. The hydrolysis temperature is 120°C, and the residence time is 10 minutes.
[0026] 3), extraction (this is as a kind of prior art): the reaction liquid is all collected after p...
Embodiment 2
[0029] Embodiment 2: a kind of production method of m-bromophenol, carries out following steps successively:
[0030] 1), diazotization reaction: with embodiment 1.
[0031] 2), hydrolysis reaction: after the above-mentioned m-bromoaniline diazosulfate aqueous solution passes through the flow pump 3, it enters the pipeline reactor 4 with a flow rate of 60ml / hr to carry out the hydrolysis reaction. The hydrolysis temperature is 120°C, and the residence time is 15 minutes.
[0032] 3), extraction: 150ml of normal hexane is placed in the extract container 70, and the extraction time is 2 hours; all the other are the same as in Example 1.
[0033] 4), product refining: take the extracted organic phase and rectify under reduced pressure, vacuum degree 30mmHg, distill out n-hexane at 22-24°C, collect fractions at 138-140°C, detect purity 98.8% by chromatography, and obtain 8.7g m-bromine phenol.
Embodiment 3
[0034] Embodiment 3: a kind of production method of m-bromophenol, carries out following steps successively:
[0035] 1), diazotization reaction: with embodiment 1.
[0036] 2), hydrolysis reaction: after the above-mentioned m-bromoaniline diazosulfate aqueous solution passes through the flow pump 3, it enters the pipeline reactor 4 with a flow rate of 160ml / hr to carry out the hydrolysis reaction. The hydrolysis temperature is 70°C, and the residence time is 3 minutes.
[0037] 3), extraction: 150ml ether is placed in the extract container 70, and the extraction time is 2.5 hours; all the other are the same as in Example 1.
[0038]4), product refinement: get the diethyl ether phase vacuum rectification that has been extracted, vacuum tightness 30mmHg, collect 138~140 ℃ fraction, through chromatographic detection purity 99%, obtain 8.5g m-bromophenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com