Application of G Protein beta subunit
A technology of G protein and subunit, applied in the application field of protein molecular marker, can solve the problems such as the correlation between the β2 subunit of G protein and hepatocellular carcinoma, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 , Preparation of protein samples from hepatocellular carcinoma tissue and paracancerous tissue
[0027] Urea, 3-[(3-cholamidopropyl)-diethylammonium]-1-propanesulfonic acid (CHAPS), phenylmethylsulfonyl fluoride (PMSF), dithiothreitol used in this example (DTT) were purchased from Sigma Company.
[0028] In this example, protein samples of cancer tissue and paracancerous tissue of hepatocellular carcinoma were prepared by nonenzymatic sample preparation (NESP), as follows:
[0029] The surgically excised fresh tissue block is quickly placed on ice and quickly cut into several small pieces that are visible to the naked eye without areas of necrosis. Use pre-cooled glutamine-free RPMI1640 medium (5% fetal bovine serum, 0.2mM PMSF, 1mM EDTA, benzisoxazole penicillin 25mg / mL, gentamicin 50mg / mL, penicillin 100 U / mL , Streptomycin 100 mg / mL, Amphotericin B 0.25 mg / mL, Nystatin 50 U / mL) After washing the tissue pieces several times, they were quickly ground into ...
Embodiment 2
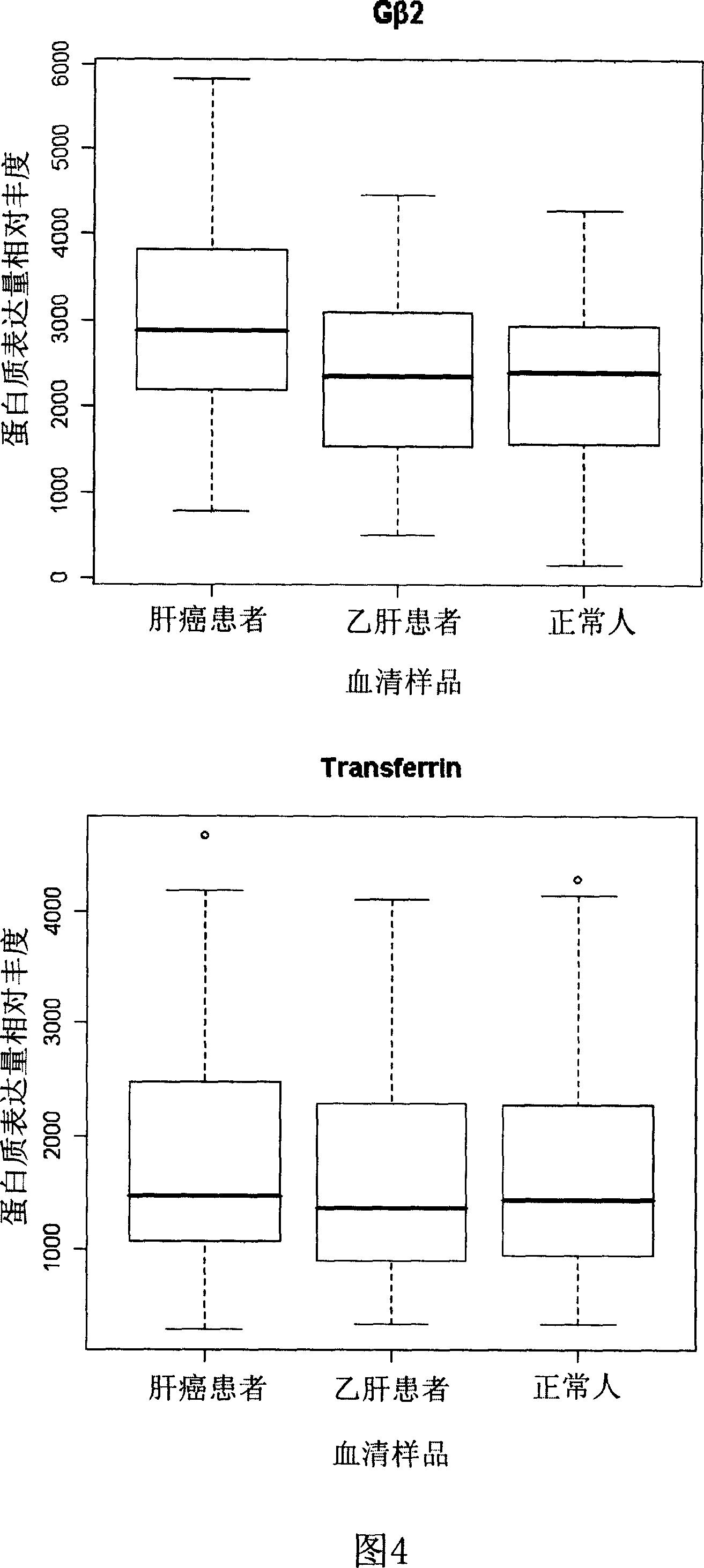
[0034] Example 2 , Screening of differentially expressed proteins
[0035] Urea, 3-[(3-cholamidopropyl)-diethylammonium]-1-propanesulfonic acid (CHAPS), and dithiothreitol (DTT) used in this example were purchased from Sigma; iodoacetamide (IAA), acrylamide, N, N-methylenebisacrylamide, etc. were purchased from Fluka.
[0036] Ammonium persulfate (AP), TEMED, Tri-n-butylphosphat (TBP), Sypro Ruby fluorescent dyes, etc. are Bio-Rad products.
[0037] LCQ™ Deca XP system and ProteomeX™ Workstation were purchased from Thermo Finnigan.
[0038] Cy2, Cy3, Cy5 fluorescent dyes, non-linear immobilized pH gradient prefabricated strips (IPG dry strips, pH3-10NL, 130×3×0.5mm), IPG buffer, IPGphore isoelectric focusing system, Amersham Pharmacia Ettan DaltII system , Typhoon scanner, automatic glue cutter, DeCyder TM Analysis software, etc. are all products of Amersham Bioscience.
[0039] Get 10 pairs (f31, f32, f33, f39, 327, 328, 415, 418, 422 and 317 in Table 1) in the 16 pairs...
Embodiment 3
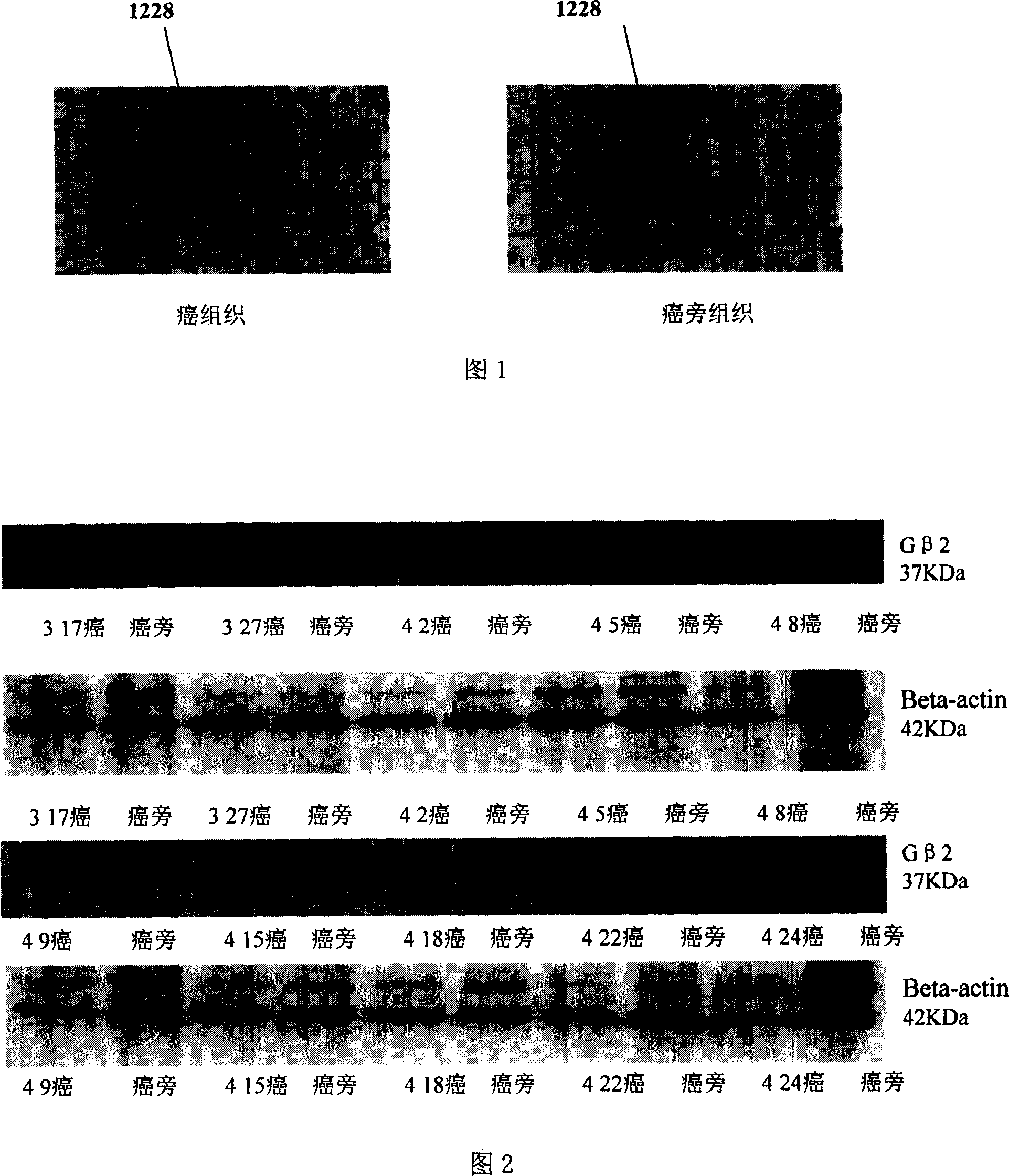
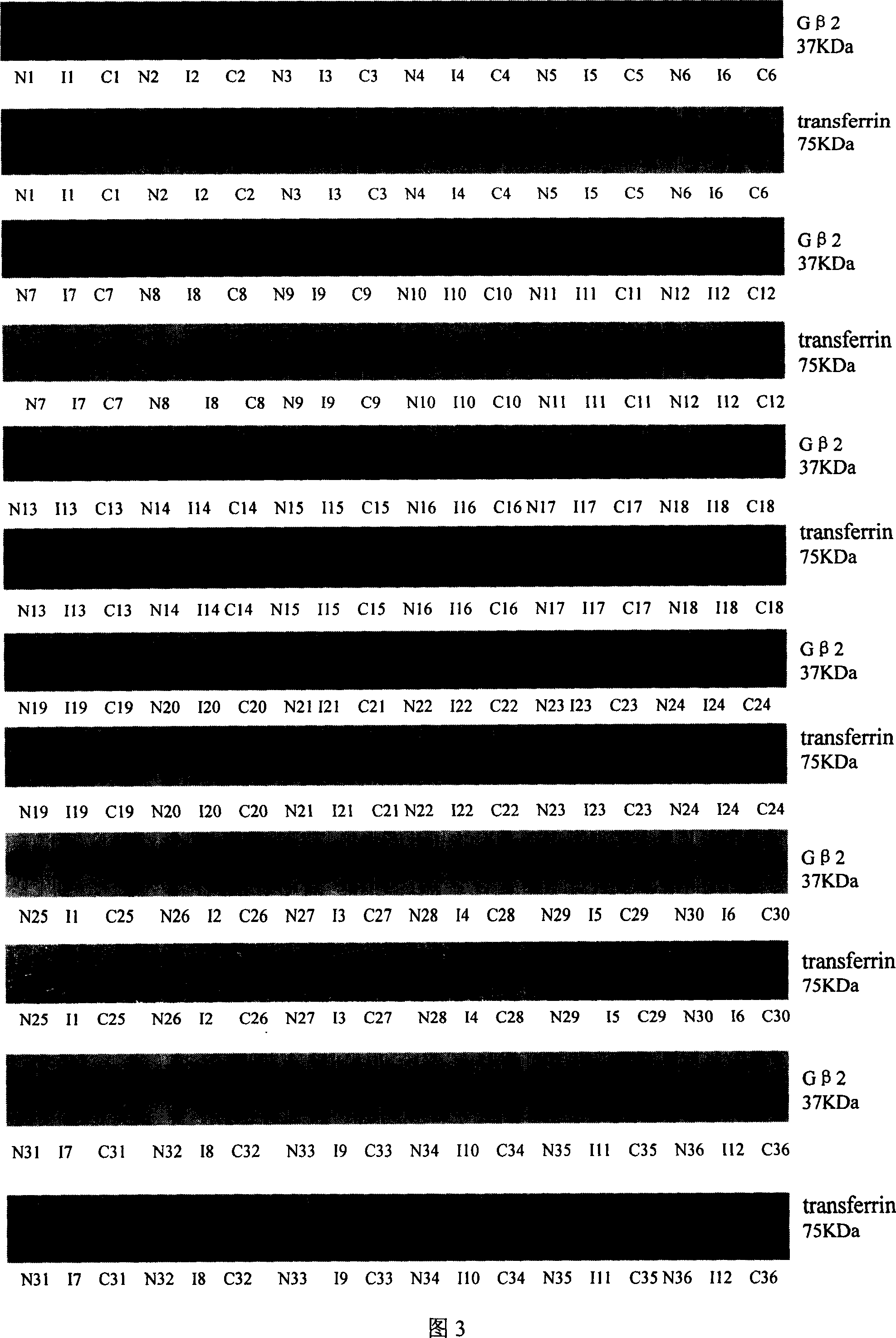
[0053] Example 3 , Western blot verification of differential expression of G protein β2 subunits
[0054] In order to confirm the differential expression of G protein β2 subunit, the protein samples of cancer tissues and corresponding paracancerous tissues of 10 patients with HCC (317, 327, 42, 45, 48, 49, 415, 418, 422 in Table 1 424), using the purchased anti-G protein β2 subunit antibody for Western blot analysis, the specific process is briefly described as follows:
[0055] Take 20 μg of protein samples from each sample and separate them with 12% SDS-PAGE, and transfer them to PVDF membranes (purchased from Amersham Biosciences), and the primary antibody uses rabbit anti-human G protein β2 subunit polyclonal antibody (purchased from Calbiochem, 1: 1000 dilution), incubated overnight at 4°C, washed three times with TBST (containing 2.42g Tris, 8g sodium chloride, 20ml Tween per liter, adjusted pH to 7.6 with HCl), 5 minutes each time, the secondary antibody was anti-rabb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com