Technique of synthesizing levorotatory betaxolol hydrochloride
A technology of betaxolol hydrochloride and synthesis process, which is applied in the field of synthesis technology of L-betaxolol hydrochloride, can solve the problems of expensive reagents and catalysts, high requirements on operation skills, low total process yield and the like, and achieves production scale Small, high optical purity, high chemical purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
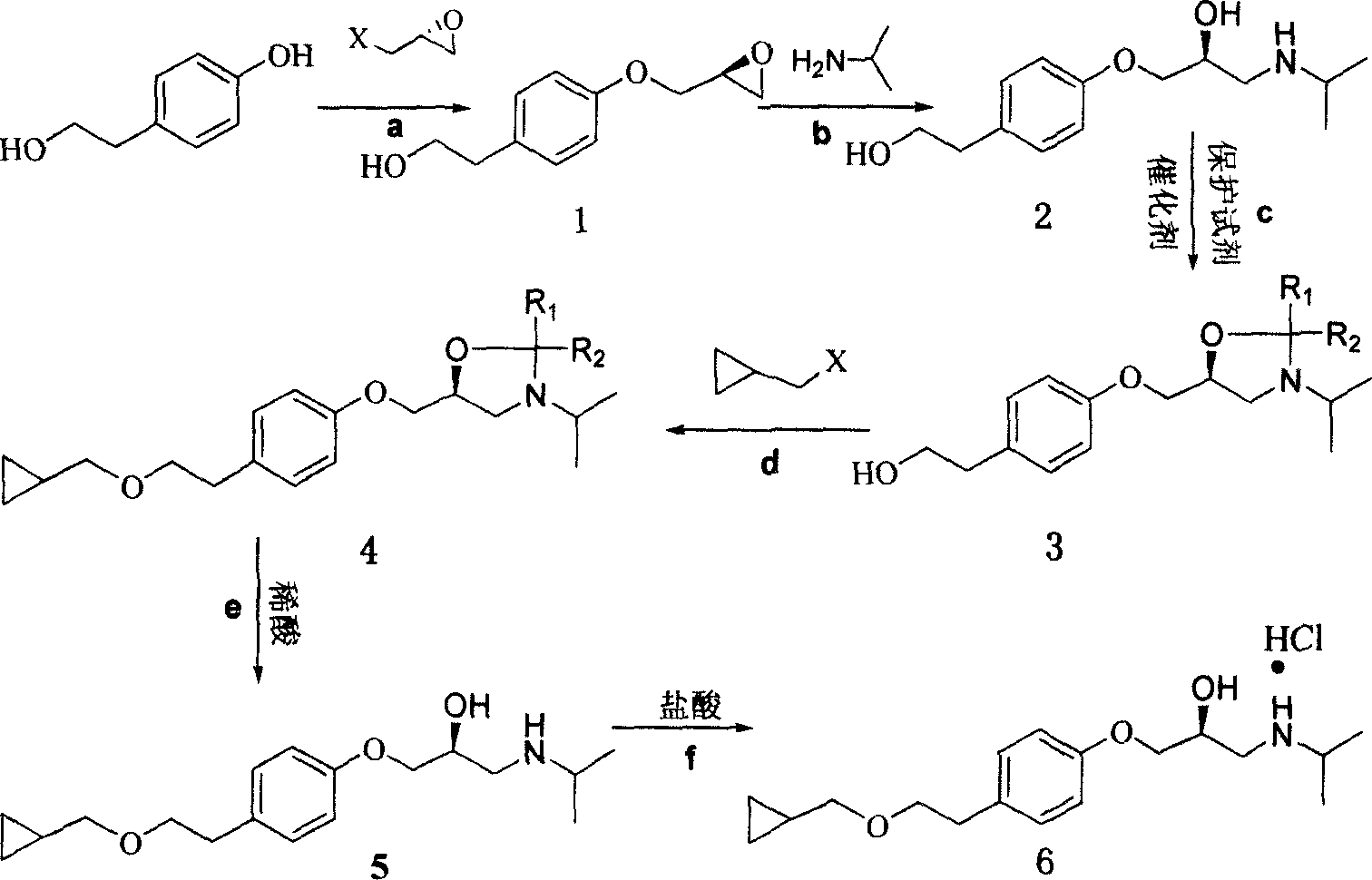
[0030] The preparation of embodiment L-betaxolol hydrochloride (compound 6)
[0031] (1) Preparation of Compound 1 (S-1-(4-(2-hydroxyethyl)phenoxy)-2,3-propylene oxide)
[0032] Dissolve p-hydroxyphenylethyl alcohol (27.60g, 0.2mol) and R-epichlorohydrin (18.50g, 0.4mol) in an organic solvent, then add sodium hydroxide (16.00g, 0.4mol) and stir mechanically, at 60°C Heated for 8 hours. After the reaction was completed, the sodium carbonate was removed by filtration, the filtrate was concentrated and evaporated to dryness, and ethyl acetate was added for recrystallization to obtain 36.10 g of S-1-(4-(2-hydroxyethyl)phenoxy)-2,3-propylene oxide , yield 93%, experimental data are as follows:
[0033] C 11 h 14 o 3 , Mp 39°C, [α] D 20 =+12.0°(c1.00, CH 3 OH); υ _ = 3349,2945,2929,2873,1613 , 1513,1245,1047,1027,906,848,823 c m - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com