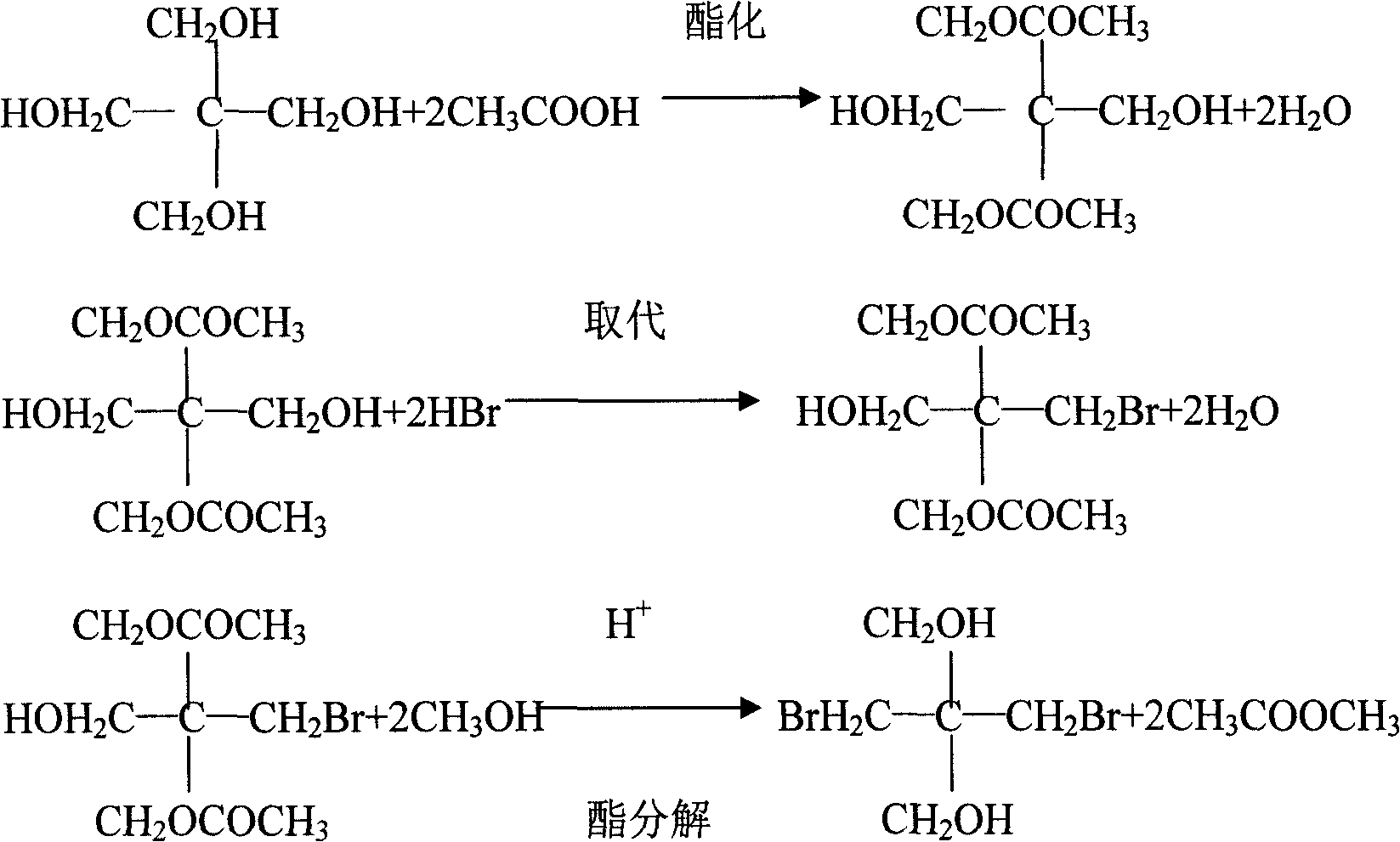Synthetic method and refining for dibromoneopentyl glycol
A technology of dibromoneopentyl glycol and synthesis method, which is applied in the field of synthesis of dibromoneopentyl glycol, can solve problems such as low product yield, high reaction temperature, and difficult separation, and achieve high product yield and high reaction temperature. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
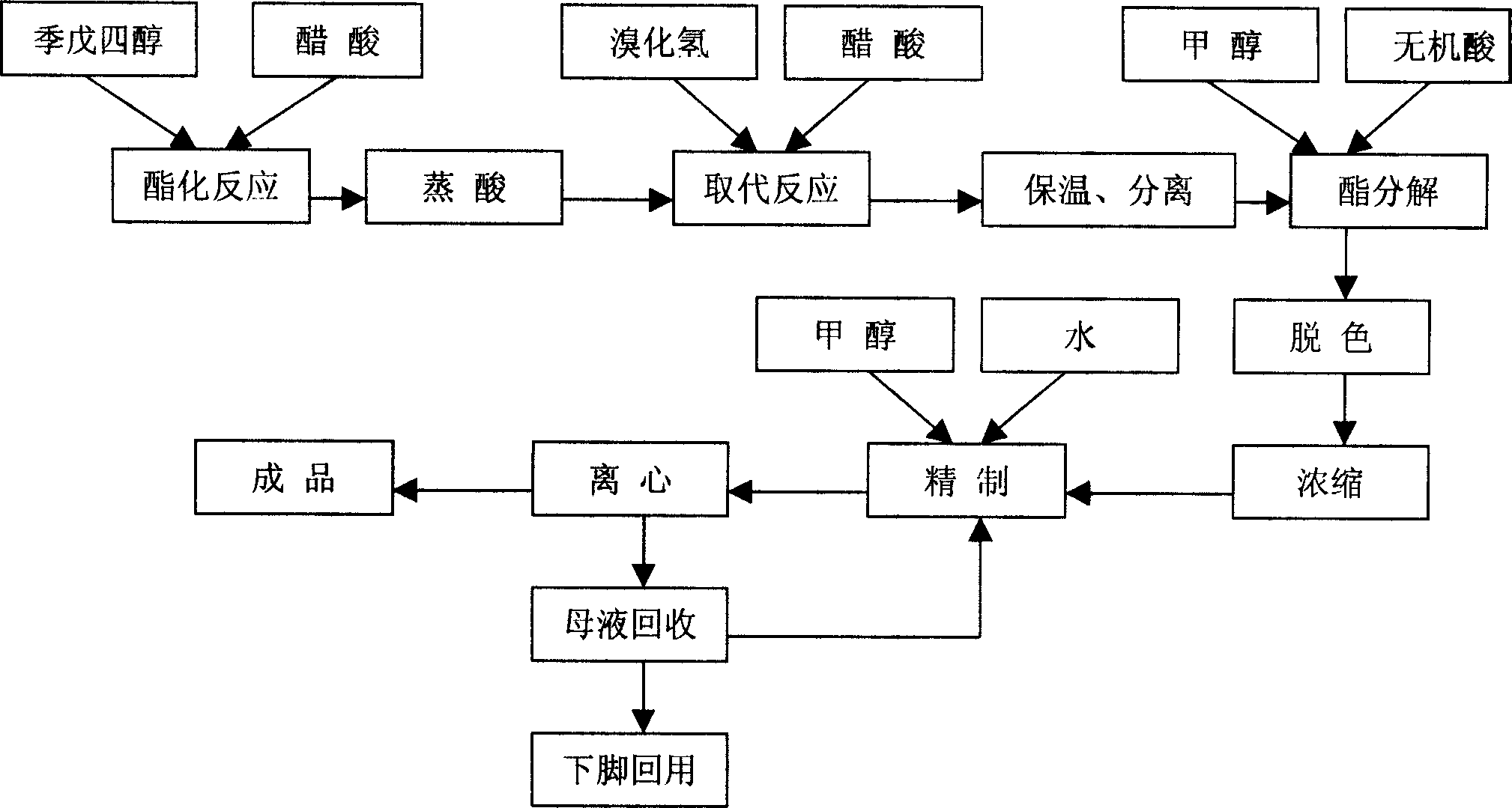
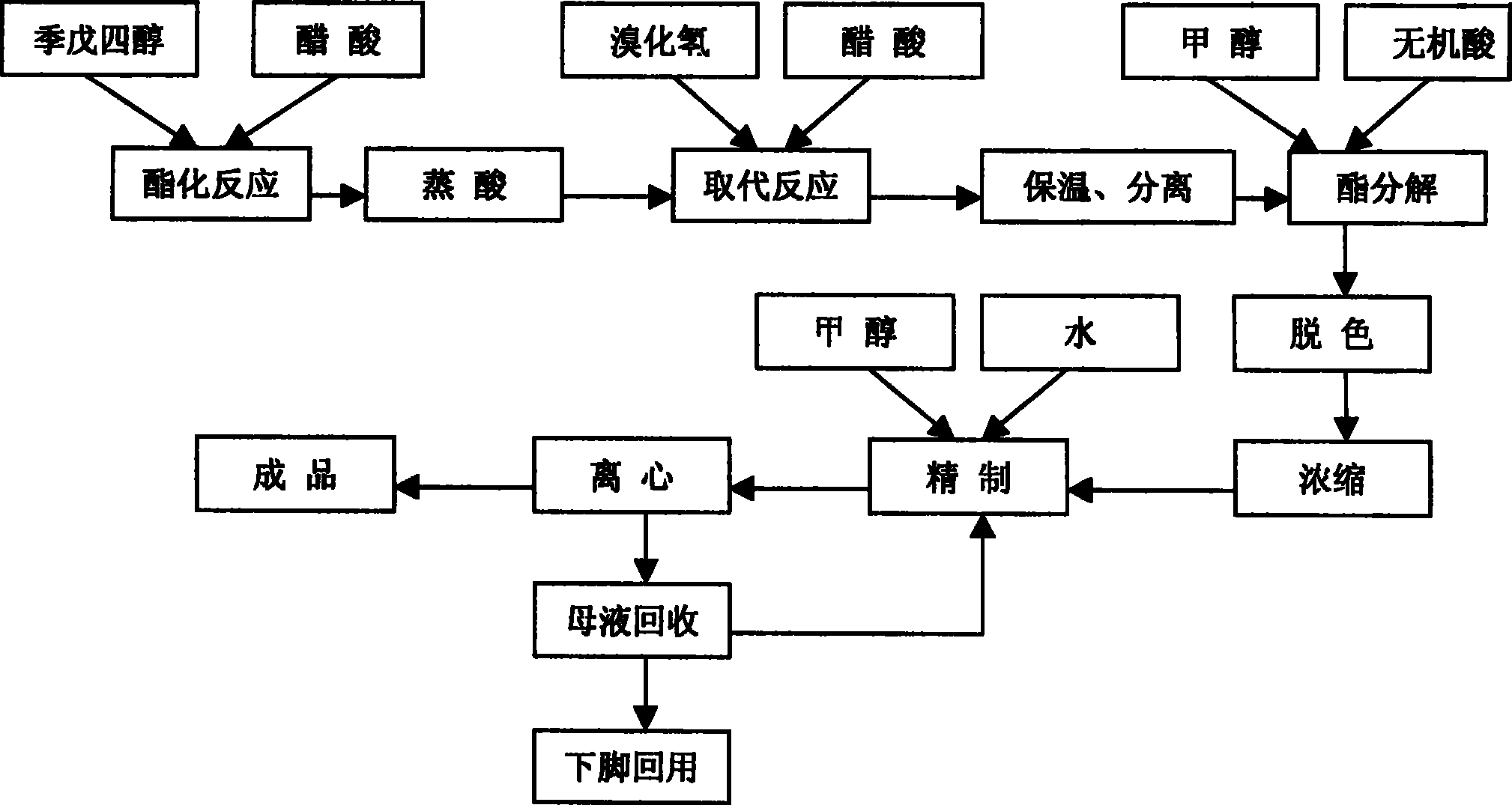
[0013] Embodiment 1, the synthetic method of dibromoneopentyl glycol, pentaerythritol is carried out esterification reaction with the acetic acid of concentration above 98%, the addition weight of acetic acid is 1.1 times of pentaerythritol weight, reflux esterification reaction 5 hours; Esterification After the reaction, the esterified product is separated by dehydration and acid distillation, the time of dehydration and acid distillation is 3.5 hours, and the temperature is 115°C; then the esterified product is dissolved with acetic acid with a concentration of more than 98%. 0.9 times, hydrogen bromide gas was introduced to carry out the substitution reaction at 85 ° C, the amount of hydrogen bromide introduced was 1.2 times the weight of pentaerythritol, the aeration time was 4 hours, and then kept at 95 ° C under normal pressure for 3.5 hours; separated after heat preservation Go out the diester of dibromoneopentyl glycol, carry out constant pressure ester decomposition un...
Embodiment 2
[0014] Embodiment 2, the synthetic method of dibromoneopentyl glycol, pentaerythritol is carried out esterification reaction with the acetic acid of concentration above 98%, the addition weight of acetic acid is 1.2 times of pentaerythritol weight, reflux esterification reaction 4 hours; Esterification After the reaction, the esterified product is separated by dehydration and acid distillation, the time of dehydration and acid distillation is controlled at 2.5 hours, and the temperature is 125°C; then the separated ester product is dissolved with acetic acid with a concentration above 98%, and the amount of acetic acid added is 1.1 times the weight, hydrogen bromide gas was introduced to carry out the substitution reaction at 105°C, the amount of hydrogen bromide introduced was 1.1 times the weight of pentaerythritol, the ventilation time was 4.5 hours, and then kept at 100°C under normal pressure for 4.5 hours; Separate the diester compound of dibromoneopentyl glycol afterward...
Embodiment 3
[0015] Embodiment 3, the synthetic method of dibromoneopentyl glycol, pentaerythritol is carried out esterification reaction with the acetic acid of concentration above 98%, the addition weight of acetic acid is 1.3 times of pentaerythritol weight, reflux esterification reaction 6 hours; Esterification After the reaction, separate the esterified product by dehydrating and distilling acid, the time of dehydrating and distilling acid is controlled to 3 hours, and the temperature is 120°C; then dissolve the separated esterified product with acetic acid with a concentration of more than 98%, and the amount of acetic acid added is 0.7 times of the weight, hydrogen bromide gas was introduced to carry out the substitution reaction at 95°C, the amount of hydrogen bromide introduced was 1.25 times the weight of pentaerythritol, the ventilation time was 5 hours, and then kept at 90°C under normal pressure for 5.5 hours; heat preservation Separate the diester of dibromoneopentyl glycol af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com