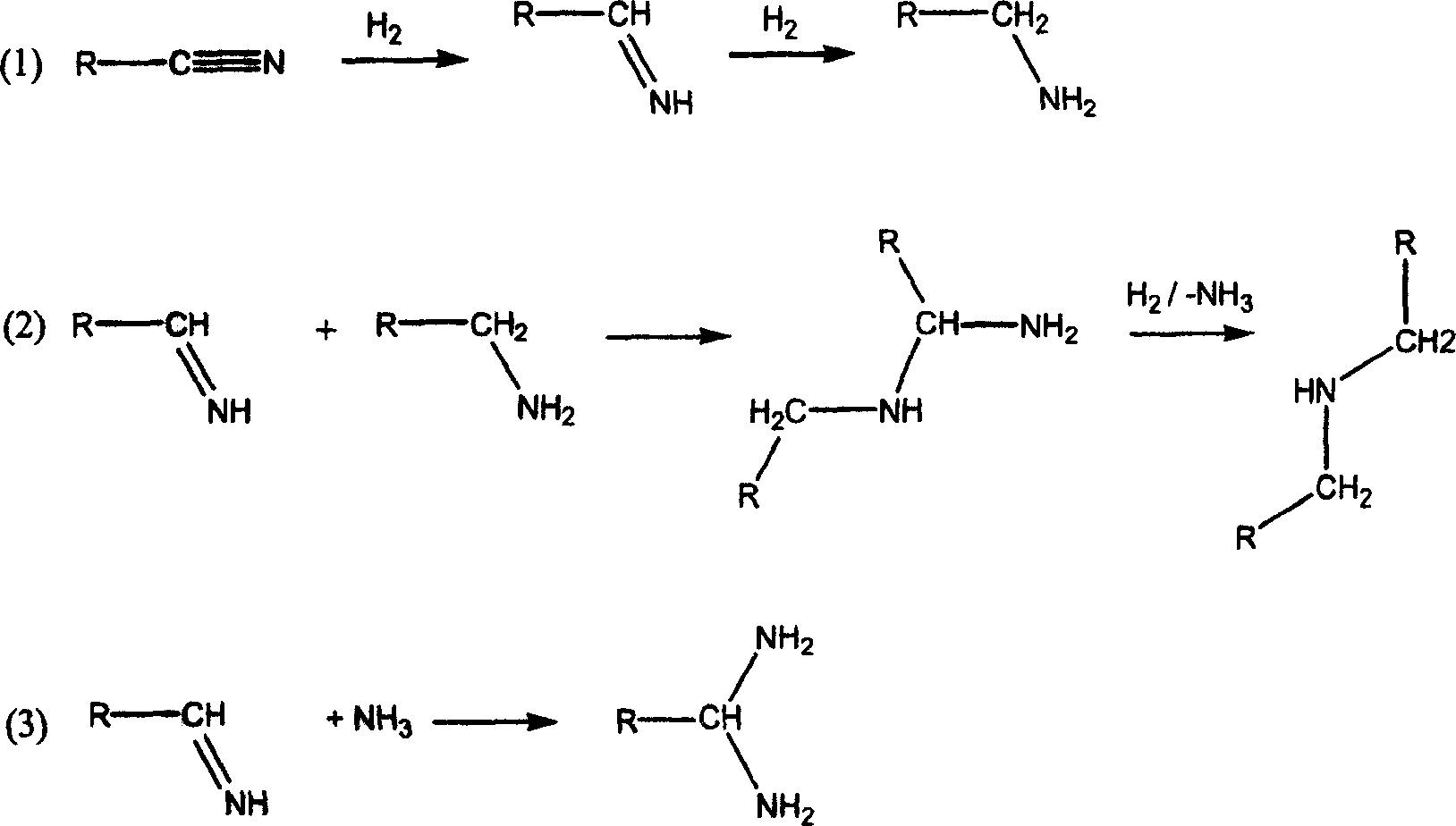
Process for preparing amines by conditioning the catalyst with ammonia
A technology of catalyst and catalyst amount, applied in catalyst activation/preparation, amino compound preparation, physical/chemical process catalyst, etc., can solve problems such as reducing catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
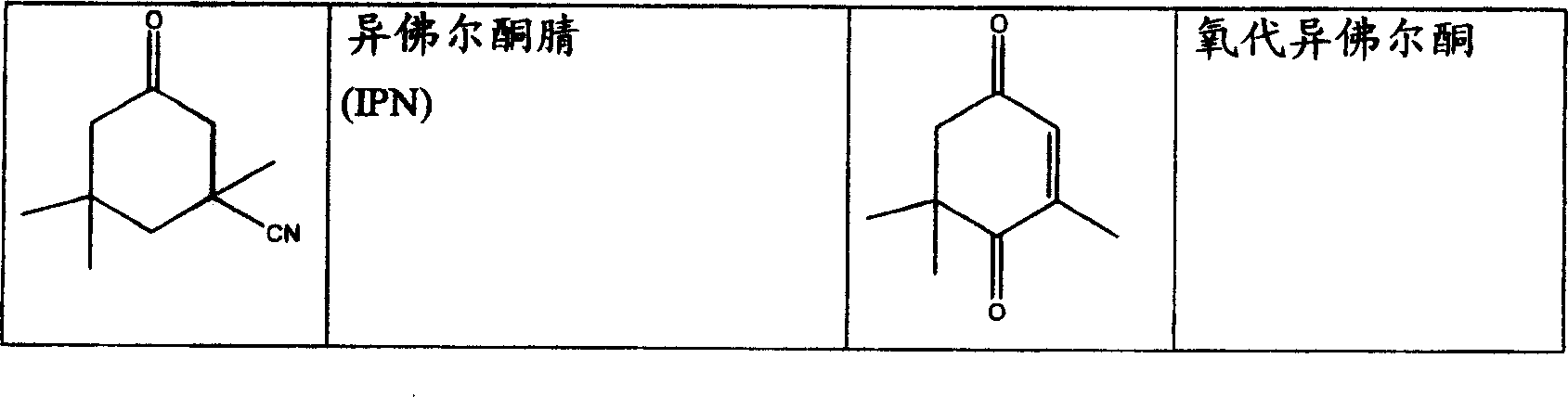
[0058] Amination and hydrogenation of 3-cyano-3,5,5-trimethylcyclohexanone (isophoronenitrile) with a cobalt-supported catalyst to obtain 3-aminomethyl-3,5,5-trimethylcyclohexanone Hexylamine (isophoronediamine).
[0059] The experimental apparatus consisted of a 100 mL fixed bed reactor for the catalyzed formation of imines by IPN and ammonia filled with an ion exchanger according to EP 042119 and a downstream connected fixed bed filled with 300 mL of cobalt catalyst in the form of small flakes (diatomaceous earth support) Reactor composition. To condition the catalyst, 300 mL / h (180 g / h) of ammonia was led through the fixed bed at a temperature of 60-100°C. During conditioning, the hydrogen partial pressure was adjusted to approximately 100 bar. Conditioning ended after two hours. Immediately after conditioning, 30 mL / h (about 28 g / h) of IPN and 400 mL / h (370 g / h) of ammonia were supplied. The two source streams can be mixed with one another immediately before the ion ex...
Embodiment 2
[0066] Amination and hydrogenation of 3-cyano-3,5,5-trimethylcyclohexanone (isophoronenitrile) with a Raney-type cobalt catalyst to obtain 3-aminomethyl-3,5,5-trimethyl Cyclohexylamine (isophorone diamine).
[0067] The experimental apparatus consisted of a 100 mL fixed bed reactor for the catalytic formation of imines from IPN and ammonia filled with an ion exchanger according to EP 042119 and a downstream connected fixed bed reactor filled with a 66 mL spherical Raney type cobalt catalyst. To condition the catalyst, 100 mL / h (60 g / h) of ammonia was led through the fixed bed at a temperature of about 100°C. During conditioning, the hydrogen partial pressure was adjusted to approximately 100 bar. Conditioning ended after two hours. Immediately after conditioning, supply 100 mL / h of a 14% solution of IPN in ammonia (LHSV=1.5h -1 ). The results of gas chromatography experiments for the product are listed in Table 2 below under the column "Conditioned".
Embodiment 3
[0074] Hydrogenation of trimethylhexamethylenedinitrile to trimethylhexamethylenediamine
[0075]The experiments were carried out in a 1 L hydrogenation autoclave equipped with a catalyst basket (static basket with stirrer, "Mahoney type". The catalyst is freshly filled.
[0076] For conditioning with ammonia, the reactor was first filled with about 500 mL of ammonia and then kept under stirring at 50° C. and 250 bar for about 2 hours. Ammonia is then evolved by depressurizing the reactor.
[0077] After conditioning, 20% to 600 mL of trimethylhexamethylene dinitrile (2,4,4-isomer and mixture of 2,2,4-isomer) at 120 °C and 250 bar total pressure The solution was hydrogenated for 6 hours. The product was drawn off and then checked by gas chromatography. The results are listed in Table 3 under the "Conditioned" column.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com