21-subunit digoxin derivative and preparation method thereof
A derivative and base technology, applied in the field of a series of digoxin derivatives and their preparation, can solve the problems of low content of natural cardiac glycoside compounds, many steps and high synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
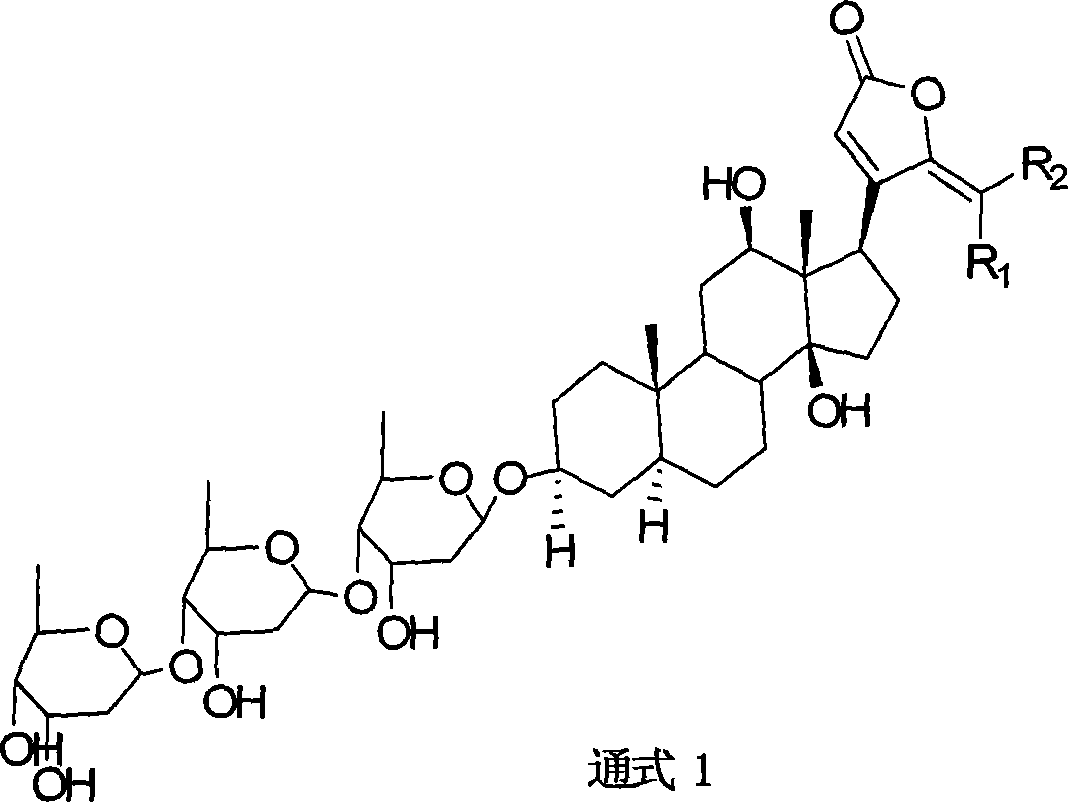
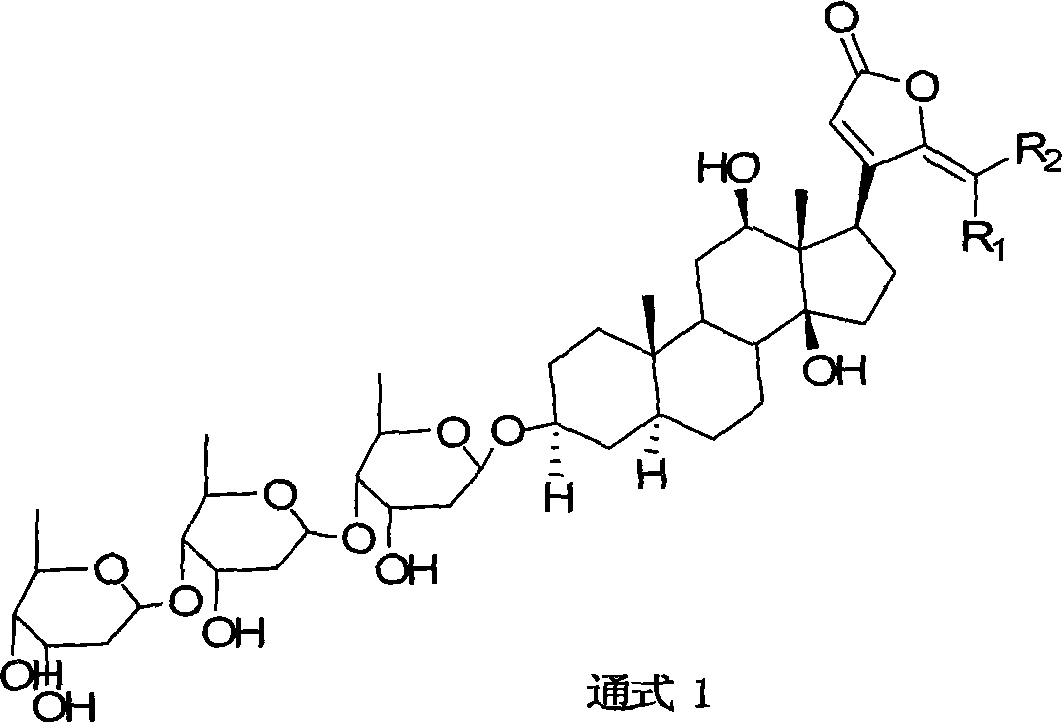
[0017] Example 1 prepares R shown in general formula 1 1 = H, R 2 = Derivatives when furyl
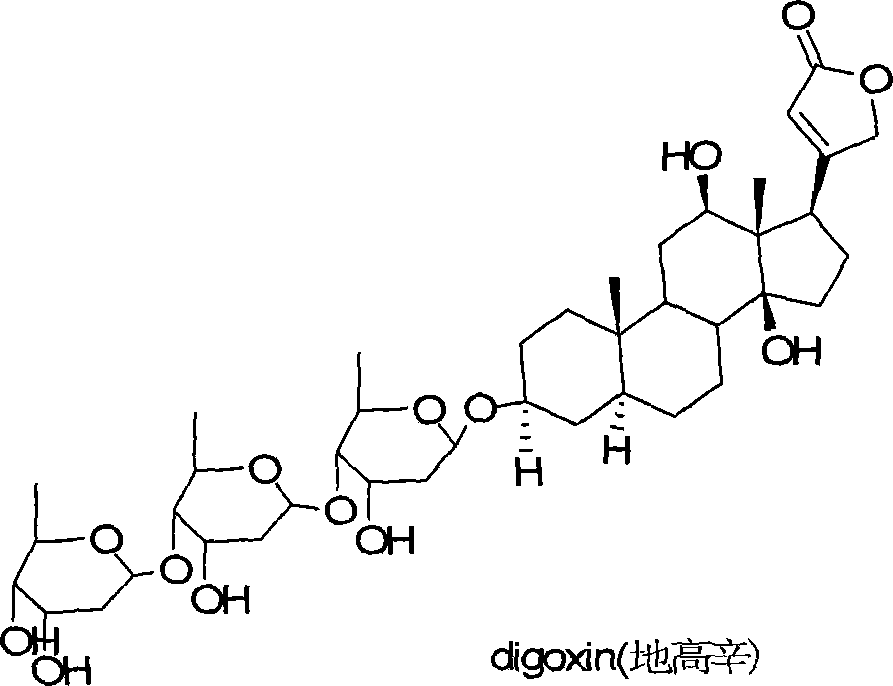
[0018] 100 mg of digoxin and 60 mg of furfural were dissolved in 10 mL of acetonitrile, refluxed for 6 h under the catalysis of triethylamine (5 mg), and the reaction was detected to be complete. After concentration, 101 mg of red solid was isolated with a yield of 88%.
[0019] mp 172.8~173.8℃; IR 3462, 2933, 2880, 1742, 1645, 1472, 1449, 1405, 1380, 1274, 1163, 1128, 1068, 1015, 953, 869cm- 1 ; 1 H NMR (400MHz, CDCl 3 , TMS): 7.74 (1H, d, J = 1.3Hz), 7.01 (1H, d, J = 3.2Hz), 6.64 (1H, s), 6.51 (1H, dd, J = 1.6, 3.2Hz), 6.17 (1H, s), 4.87~4.92(3H, om), 4.2(2H, t, J=3.7Hz), 4.1(2H, m), 4.03(1, s), 3.75~3.78(4H, om), 3.48(1H, m), 3.75(1H, m), 3.2~3.3(4H, m), 2.25~1.65(20H, om), 1.27(6H, om), 1.23(9H, om), 0.91(3H, s), 0.77(3H,om); 13 C NMR (100.6MHz, CDCl 3 , TMS): δ170.2, 164.4, 149.5, 148.4, 143.8, 115.6, 114.9, 112.9, 100.1, 98.4, 98.3, 95.5, 86.5, 82.6, 28.2, 75.7, 72.7, 72....
Embodiment 2
[0020] Embodiment 2 prepares general formula R 1 = H, R 2 = Derivatives of p-methoxyphenyl
[0021] 100 mg of digoxin and 60 mg of anisaldehyde were dissolved in 15 mL of tetrahydrofuran, refluxed for 5 h under the catalysis of pyridine (5 mg), and the reaction was detected to be complete. After concentration, 88 mg of light yellow solid was isolated with a yield of 80%.
[0022] mp 156.5~157.8℃; IR3455, 2932, 2880, 1737, 1604, 1511, 1449, 1406, 1379, 1302, 1255, 1175, 1128, 1061, 1014, 954, 868, 829cm- 1 ; 1 H NMR (400MHz, CDCl 3 , TMS): 7.75 (1H, d, J = 8.4Hz), 6.89 (1H, d, J = 8.6Hz), 6.6 (1H, s), 6.15 (1H, s), 4.85 ~ 4.92 (); 13 C NMR (100.6MHz, CDCl 3 , TMS): δ170.2, 164.4, 149.5, 148.4, 143.8, 13H, m1, 4.2 (2H, m), 4.1 (1H, m), 4.02 (1H, s), 3.82 (3H, s), 3.84~ 3.75(4H, m), 3.50(2H, m), 3.19~3.27(4H, m), 2.25~1.45(20H, om), 1.27(6H, om), 1.23(9H, om), 0.91(3H, s), 0.77(3H, s); 13 C NMR (100.6MHz, CDCl 3 , TMS): δ170.8, 165.2, 160.1, 148.7, 132.5, 126.2, 114.6, 1...
Embodiment 3
[0023] Embodiment 3: preparation general formula R 1 = H, R 2 =CHCCH 3 CH 2 CH 2 CHC (CH 3 ) 2 time derivative
[0024] 100 mg of digoxin and 60 mg of citral were dissolved in 15 mL of ethanol, under the catalysis of potassium carbonate (5 mg), the reflux reaction was carried out for 7 hours, and the reaction was detected to be complete. After concentration, 76 mg of light yellow solid was isolated with a yield of 65%.
[0025] mp 159.7~160.4℃; IR3453, 2966, 2933, 2880, 1734, 1635, 1578, 1449, 1405, 1378, 1316, 1273, 1164, 1128, 1068, 955, 869cm- 1 ; 1 H NMR (400MHz, CDCl 3, TMS): 6.63(1H, d, J=12.0Hz), 6.40(1H, d, J=11Hz), 6.08(1H, s), 5.01(1H, s), 4.90~4.75(4H, om), 4.25(2H, br), 4.11(1H, s), 4.02(1H, s), 3.73~3.77(6H, om), 3.44~3.47(2H, m), 3.22~3.29(5H, m), 3.1( 2H, br), 2.16~1.47(33H, om), 1.27(6H, om), 1.23(9H, om), 0.92(3H, s), 0.75(3H, s); 13 CNMR (100.6MHz, CDCl 3 , TMS): δ170.5, 163.7, 149.1, 147.8, 123.7, 118.6, 115.0, 109.3, 98.3, 98.2, 95.4, 86.5, 82.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com