Method for enriching and sequencing protein terminal peptide fragment and reagent kit
A protein and kit technology, applied in the field of protein sequencing, to achieve a wide range of practical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
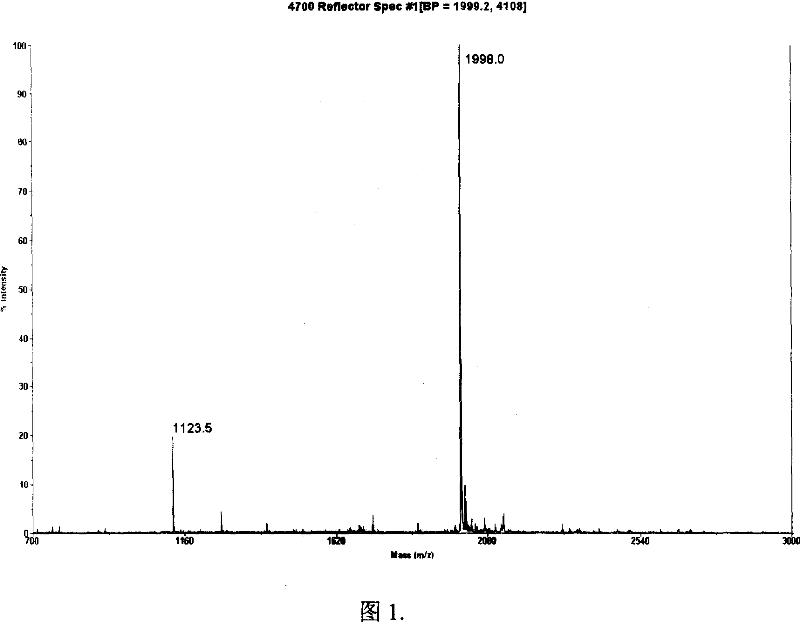
[0032] Example 1 Enrichment and sequencing of terminal peptides in horse myoglobin
[0033] 1.1 Dissolve 1 mg of horse myoglobin in 1 mL of 0.2M borate solution at pH 8, add 20 mM o-sulfobenzoic anhydride, react at room temperature for 1 hour, then add 100 mM Tris / HCl to terminate the reaction. Myoglobin does not contain cysteine residues, so the reductive alkylation step can be omitted. Add 20ug trypsin, digest at 37°C for 12 hours.
[0034] 1.2 On the Elite P230 liquid chromatograph (Dalian Elite Company), use a C18-reversed-phase chromatographic column (200×4.6mm, 5um, 300 Ȧ, Dalian Elite Company) and a strong cation exchange column (200×4.6 mm, 5um, 300 Ȧ, Dalian Elite Company) in series for the enrichment of terminal peptides. Equilibrate the chromatographic system with 0.1% trifluoroacetic acid (TFA) first, wash the column with 10 times the column volume of 0.1% trifluoroacetic acid (TFA) for desalting after loading the sample, then wash the column with 60% methanol ...
Embodiment 2
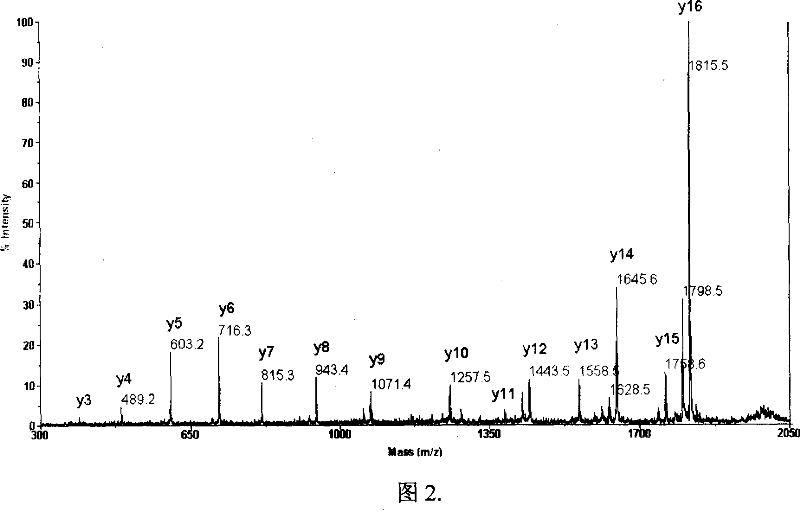
[0036] Example 2 Enrichment and sequencing of bovine lactoglobulin terminal peptides
[0037]2.1 Take 1mg bovine lactoglobulin, dissolve it in 0.2mL 0.2M pH 8 borate solution (containing 6M guanidine hydrochloride), add 20mM o-sulfobenzoic anhydride, react at room temperature for 1 hour, then add 100mM Tris / HCl to stop reaction. Add 10mM dithiothreitol (DTT), react at 37°C for 1 hour, dilute 6 times with water, add 20ug trypsin, and digest at 37°C for 12 hours.
[0038] 2.2 On the Elite P230 liquid chromatograph (Dalian Elite Company), use a C18-reversed-phase chromatographic column (200×4.6mm, 5um, 300 Ȧ, Dalian Elite Company) and a strong cation exchange column (200×4.6 mm, 5um, 300 Ȧ, Dalian Elite Company) in series for the enrichment of terminal peptides. Equilibrate the chromatographic system with 0.1% trifluoroacetic acid (TFA) first, wash the column with 10 times the column volume of 0.1% trifluoroacetic acid (TFA) for desalting after loading the sample, and then wash...
Embodiment 3
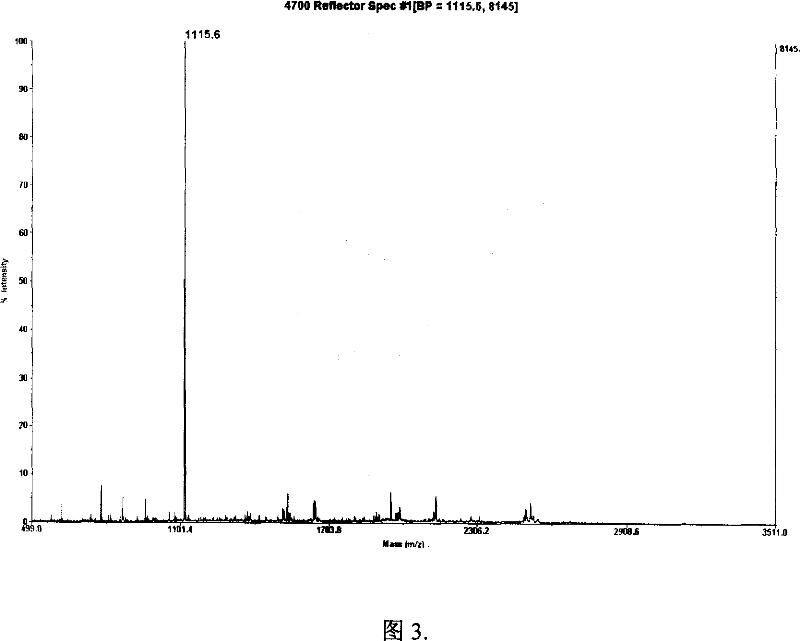
[0040] Example 3 Enrichment and sequencing of bovine insulin terminal peptide
[0041] 3.1 Take 1mg bovine insulin, dissolve it in 0.2mL 0.2M pH 8 borate solution (containing 6M guanidine hydrochloride), add 20mM o-sulfobenzoic anhydride, react at room temperature for 1 hour, then add 100mM Tris / HCl to stop the reaction . Add 10mM dithiothreitol (DTT), react at 37°C for 1 hour, dilute 6 times with water, add 20ug trypsin, and digest at 37°C for 12 hours.
[0042] 3.2 On the Elite P230 liquid chromatograph (Dalian Elite Company), use a C18-reversed-phase chromatographic column (200×4.6mm, 5um, 300 Ȧ, Dalian Elite Company) and a strong cation exchange column (200×4.6 mm, 5um, 300 Ȧ, Dalian Elite Company) in series for the enrichment of terminal peptides. Equilibrate the chromatographic system with 0.1% trifluoroacetic acid (TFA) first, wash the column with 10 times the column volume of 0.1% trifluoroacetic acid (TFA) for desalting after loading the sample, then wash the column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com