S-type clopidogrel and preparation method of its salt
A type of clopidogrel and hydrochloride technology, applied in the field of compound preparation, can solve the problems of affecting the quality of clopidogrel, long time required, great toxicity, etc., saving operation time and labor cost, and simple and time-saving operation. , difficult to control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
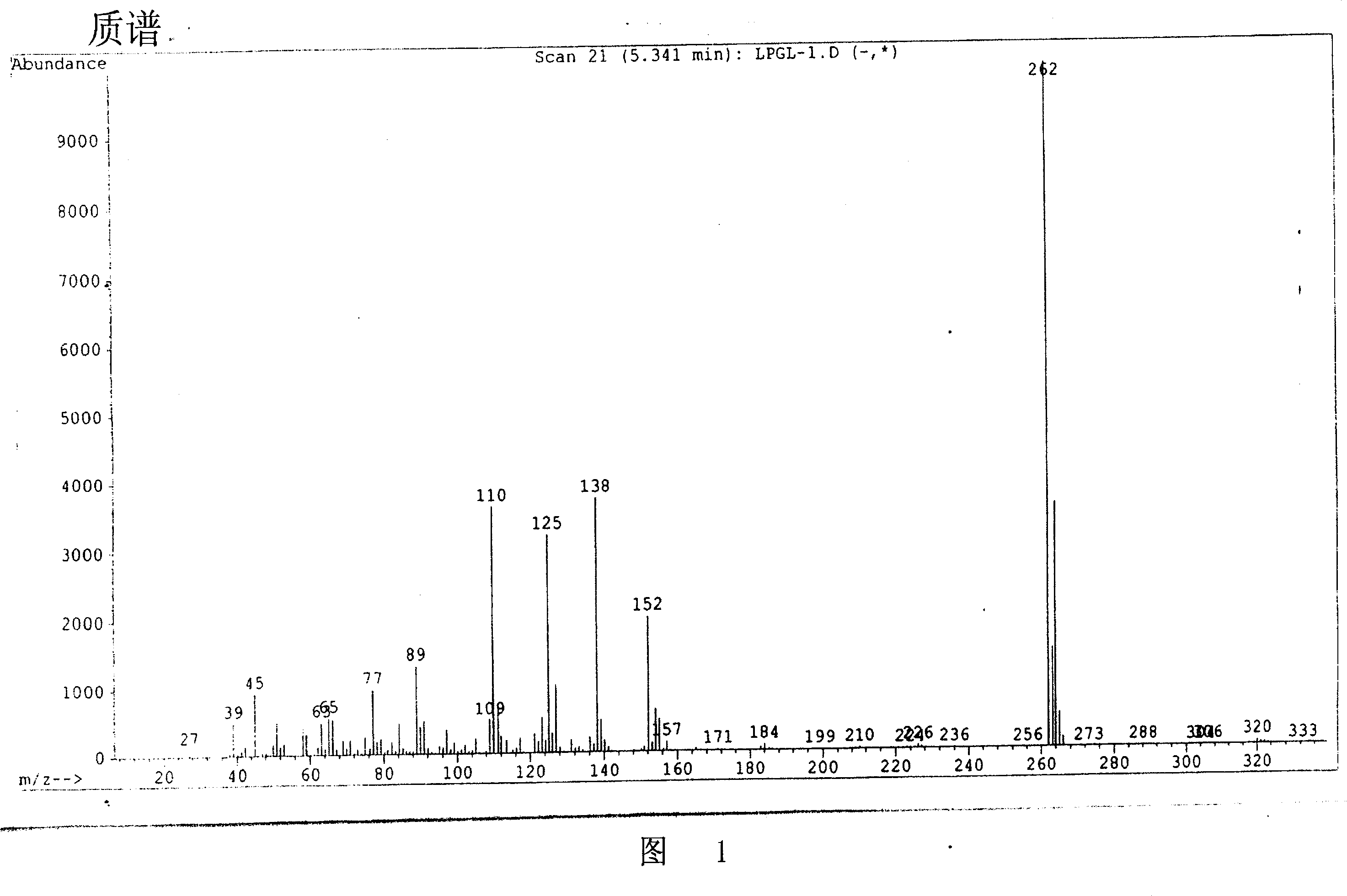
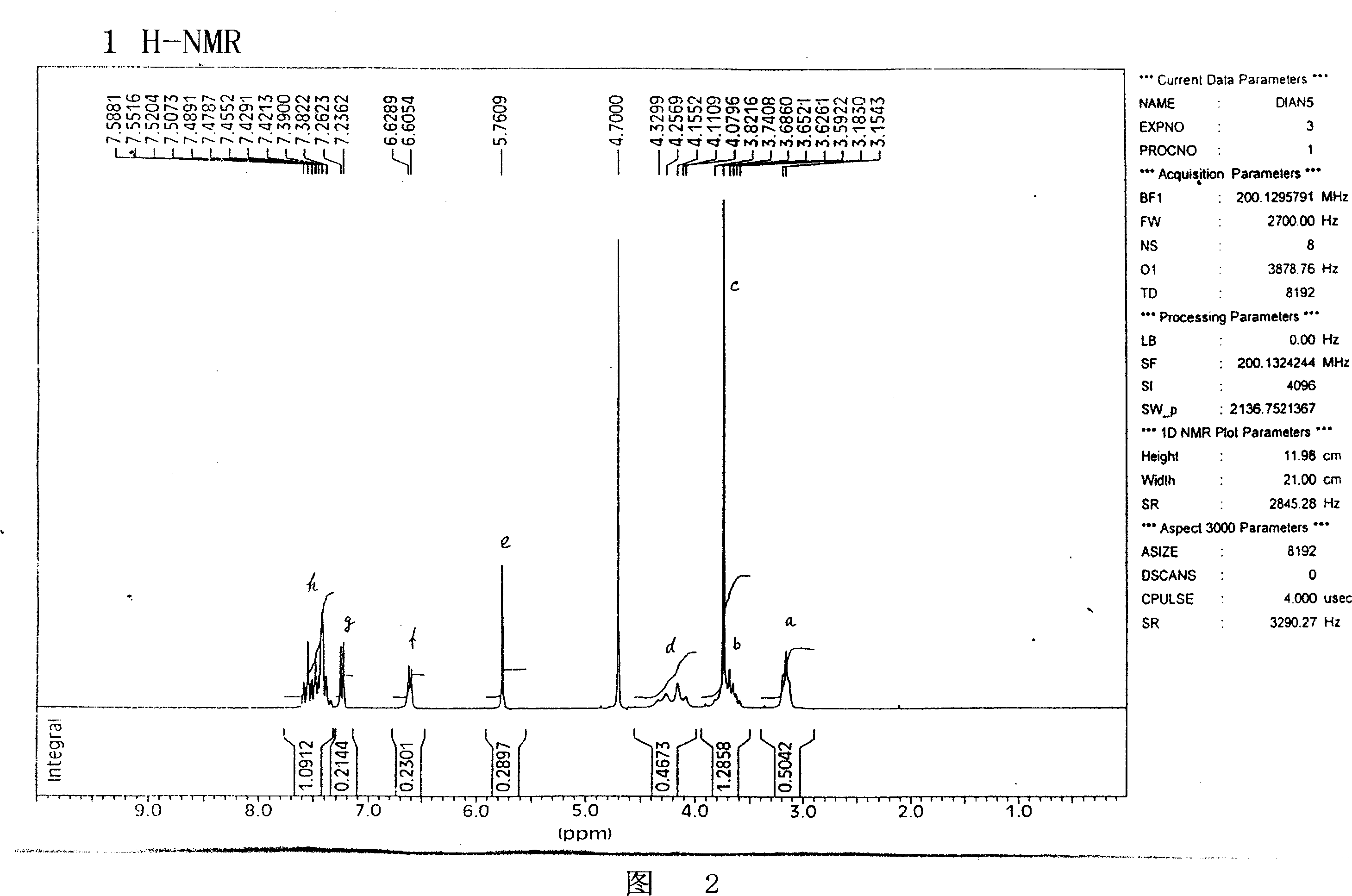
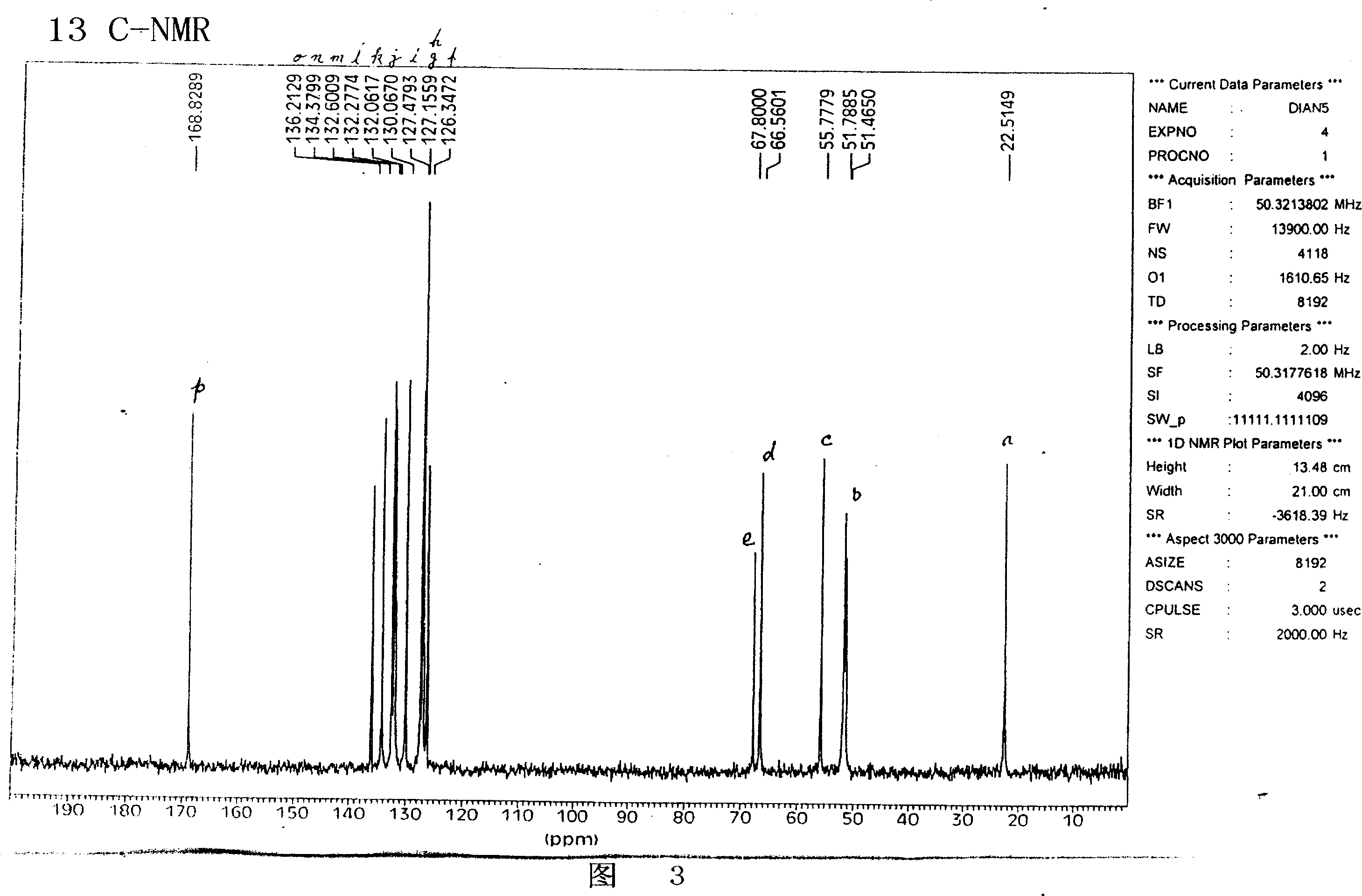
Image
Examples
Embodiment 1
[0044] Weigh 14g (0.040mol) of racemic α-(2-thienylethylamino)-α-(2-chlorophenyl) methyl acetate hydrochloride, add 30mL water and 90mL dichloromethane, stir to Adjust the pH to 7 with sodium bicarbonate, discard the aqueous layer after separation, wash the dichloromethane layer with 30 mL of water three times, dry over sodium sulfate and evaporate the solvent under reduced pressure to obtain 12 g (0.039 mol) of an oily substance.
[0045] After the oil was dissolved in 150 mL of isopropanol, 4.9 g (0.033 mol) of L-tartaric acid was added, stirred and kept at 50°C for 0.5 hours, and when the temperature was naturally lowered to 35°C, seed crystals were added, stirred overnight at room temperature, cooled to 20°C and stirred for 4 Hours, the solid was filtered, washed and dried to obtain the product, optical rotation: [α] 20 =+88°~+89°. Weigh 50g of the split (+)II-L-tartrate, add 200mL of isopropanol and stir to dissolve at 65°C, cool down and precipitate naturally, stir at 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com