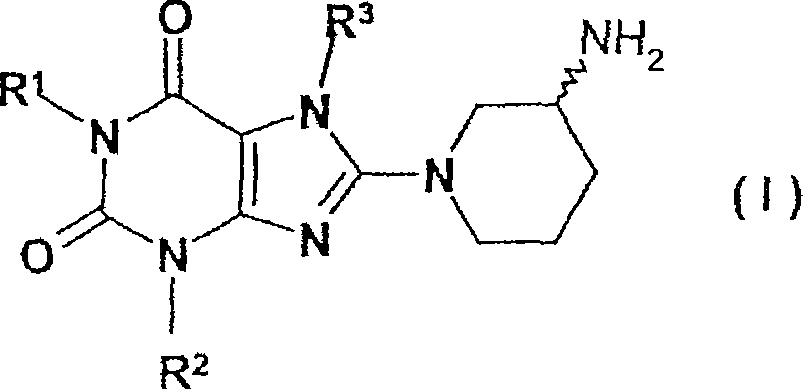
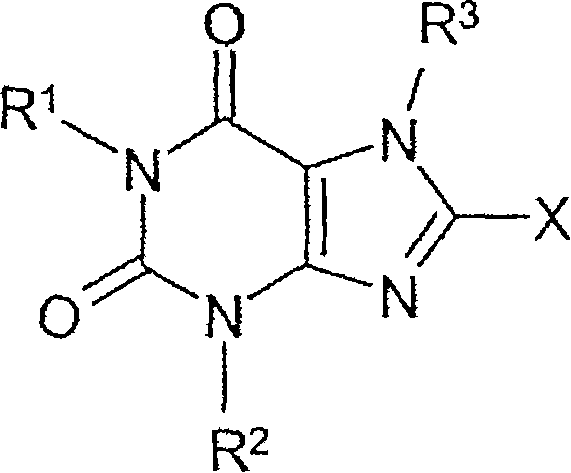
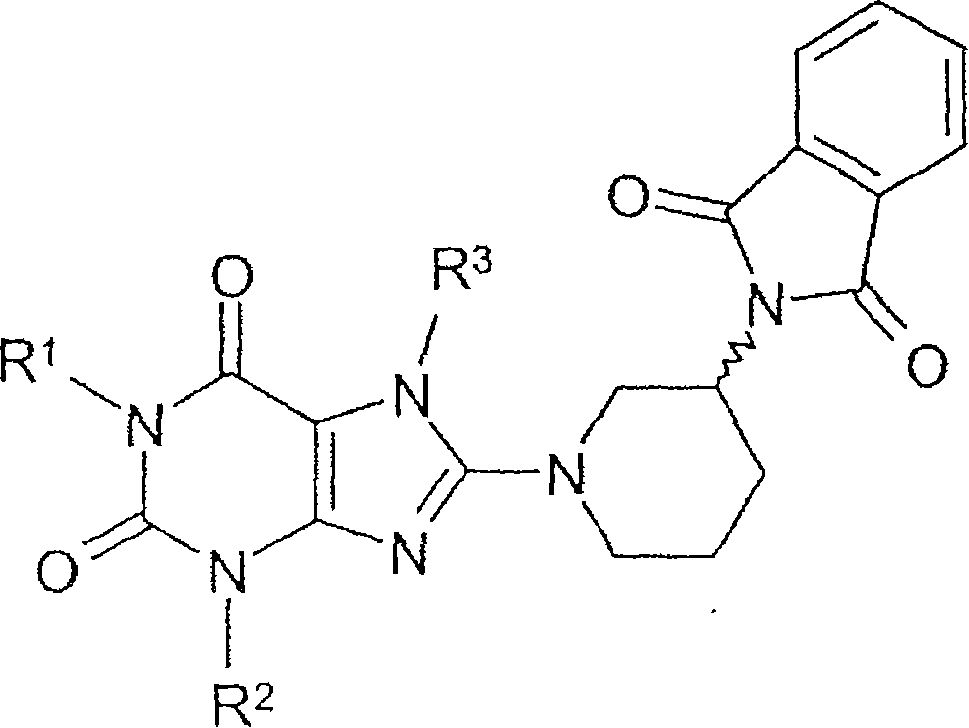
Method for producing chiral 8-(3-amino-piperidin-1-yl)-xanthines
A technology of piperidine and naphthylmethyl, applied in the field of preparing chiral 8--xanthine, can solve problems such as unfavorable applicability of 8-(3-aminopiperidin-1-yl)-xanthine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] D-tartrate of the R enantiomer of 3-(phthalimido)piperidine
[0040] a. Hydrogenation:
[0041]
[0042] 10.00kg (106.25mol) of 3-aminopyridine, 500g of industrial-grade activated carbon and 65 liters of acetic acid were first charged into the hydrogenation reactor. 50 g of Nishimura catalyst (commercially available rhodium / platinum hybrid catalyst) was slurried in 2.5 liters of acetic acid and flushed in 2.5 liters of acetic acid. Hydrogenation was performed at 50° C. under a hydrogen pressure of 100 bar until hydrogen uptake ceased, followed by post-hydrogenation at 50° C. for 30 minutes. The catalyst and charcoal were filtered off and washed with 10 L of acetic acid. The product solution was subjected to further reactions without purification.
[0043] The reaction can also be performed under less stringent pressures.
[0044] b. Acylation
[0045]
[0046] Firstly, 15.74kg (106.25mol) of phthalic anhydride was charged into the reactor. and mixed with the f...
Embodiment 2
[0054] Synthesis of 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-(R)-aminopiper Pyridin-1-yl)-xanthine
[0055] a.2-Chloromethyl-4-methylquinazoline
[0056]
[0057] First charge 10.00 kg (73.98 mol) of 2-aminoacetophenone and add 24.5 liters of 1,4-dioxane. The solution was cooled to 10°C and mixed with 16.72 kg (458.68 mol) of hydrogen chloride introduced. The reaction mixture was warmed to 22-25°C. Hydrogen chloride was reintroduced at this temperature. At about half of the total charge, the mixture was cooled to -10°C and the charge continued. Subsequently, the resulting suspension was left at -10°C overnight. A solution of 6.70 kg (88.78 mol) of chloroacetonitrile in 2.5 liters of 1,4-dioxane was added within one hour at -10°C. The feed vessel was flushed with 2 liters of 1,4-dioxane. Afterwards, the contents of the reactor were warmed to 6°C and stirred for an additional approximately 2 hours.
[0058] A mixture of 122 liters of water an...
Embodiment 3
[0076] 1-[(3-cyano-pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-(R)-amino-piperidine -1-yl)-xanthine
[0077] a. 3-cyano-2-(chloromethyl)-pyridine
[0078] 165.5 g (0.98 mol) of 2-hydroxymethyl-3-pyridinecarboxamide (pyridinecarboxamide) and 270 ml of phosphorus oxychloride (phosphorus oxychloride) were heated at 90-100° C. for 1 hour. The reaction mixture was cooled to room temperature and then about 800 ml of water was added dropwise at 50-60°C. After the phosphorus oxychloride is hydrolyzed, it is neutralized with sodium hydroxide solution under cooling, and the product is precipitated during this period. It was filtered, washed with 300 ml of water and then dried at 35-40°C.
[0079] Yield: 122.6 g (82% of theory)
[0080] Alternative to step a: 3-cyano-2-(chloromethyl)pyridine
[0081] 20.0 g (131.45 mmol) of 2-hydroxymethyl-3-pyridinecarboxamide were suspended in 110 ml of acetonitrile and heated to 78°C. Within 15 minutes, 60.65 g (395.52 mmol) of phosphoru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com