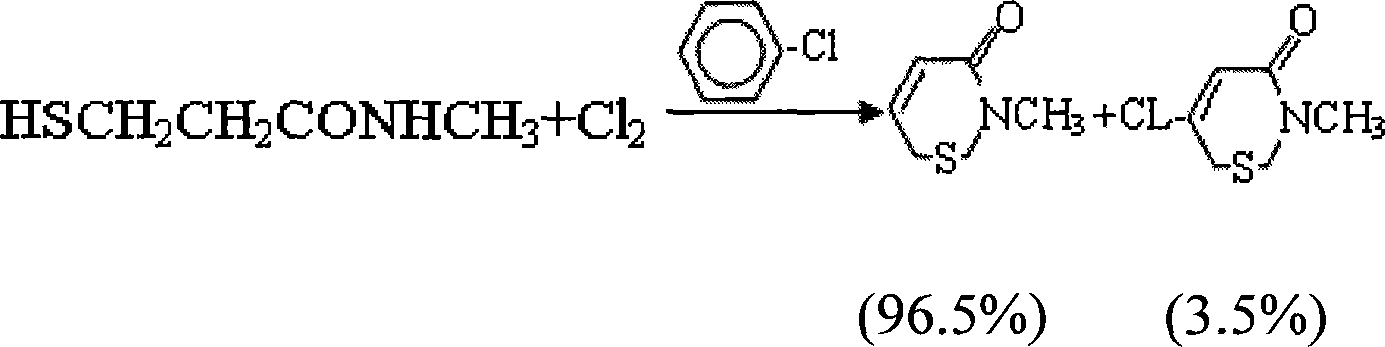
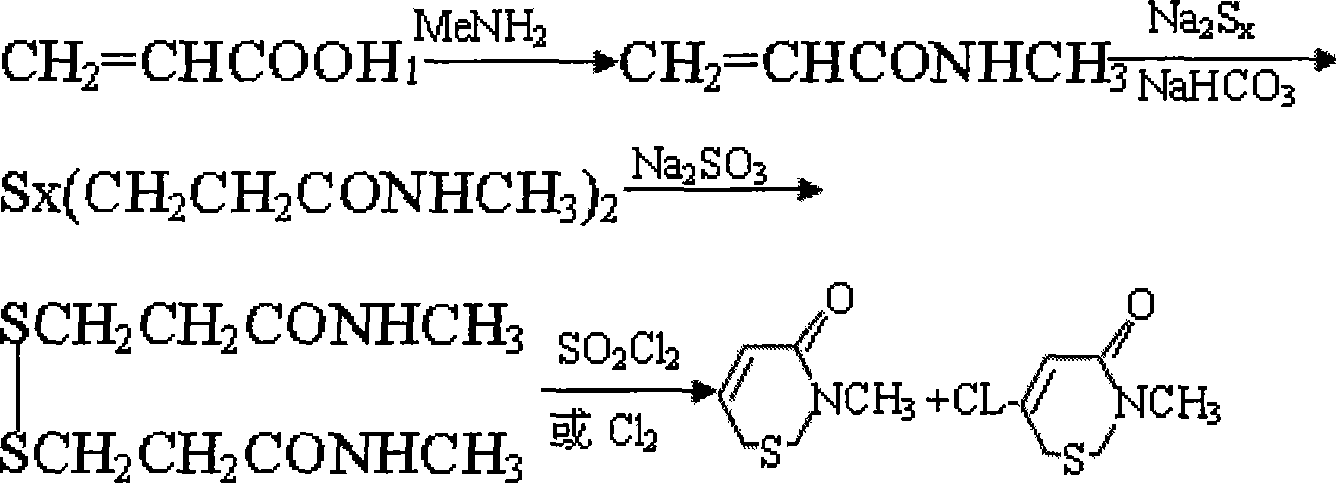Method for preparing new type bactericide of iso-thiazolinone
A technology of isothiazolinone and fungicide, applied in the field of compound preparation, can solve problems such as difficult to remove, unfavorable ring closing reaction, difficult to recycle, etc., to achieve the effect of increasing yield, reducing side reactions, and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of dimethyl disulfide dipropionate
[0032] Add 84 grams of sodium bicarbonate to 86 grams of methyl acrylate and stir, cool in an ice bath to 0-15 ° C, dropwise add 300 grams of sodium polysulfide solution with a concentration of 40%, and after the addition is complete, continue stirring at room temperature for 5 After 1 hour, the lower layer product was separated from the upper layer aqueous solution, 500 grams of saturated sodium sulfite solution was added to the product, heated and stirred at 50 ° C for 3 hours, the reaction product was separated from the water phase again, separated and purified to obtain 192 grams of dimethyl disulfide dipropionate , the yield is 81%;
[0033] (2) Preparation of N, N'-dimethyldithiodipropionamide
[0034] Add 700 ml of N,N-dimethylformamide solution of methylamine dropwise to 192 g of dimethyl disulfide dipropionate, the temperature of the addition is controlled at 0-5°C, and after the addition, the temperature is...
Embodiment 2
[0038] (1), the preparation of dimethyl disulfide dipropionate
[0039] Raw material addition is identical with embodiment 1.
[0040] Add sodium bicarbonate to methyl acrylate for stirring, cool in an ice bath to 0-15°C, add sodium polysulfide solution dropwise, after the dropwise addition is complete, continue to stir and react at room temperature for 3 hours, separate the lower layer product from the upper layer aqueous solution, and Sodium sulfite solution was added to the product, heated and stirred at 50° C. for 3 hours, the reaction product was separated from the water phase again, separated and purified to obtain dimethyl disulfide dipropionate; the amount of raw materials added was the same as in Example 1.
[0041] (2) Preparation of N, N'-dimethyldithiodipropionamide
[0042] Raw material addition is identical with embodiment 1.
[0043] Add the N,N-dimethylformamide solution of methylamine dropwise to dimethyl disulfide dipropionate, the dropping temperature is c...
Embodiment 3
[0048] (1), the preparation of dimethyl disulfide dipropionate
[0049] Raw material addition is identical with embodiment 1.
[0050] Add sodium bicarbonate to methyl acrylate for stirring, cool in an ice bath to 0-15°C, add sodium polysulfide solution dropwise, after the dropwise addition is complete, continue to stir and react at room temperature for 3 hours, separate the lower layer product from the upper layer aqueous solution, and Add sodium sulfite solution to the product, heat and stir at 50°C for 3 hours, separate the reaction product from the water phase again, separate and purify to obtain dimethyl disulfide dipropionate;
[0051] (2) Preparation of N, N'-dimethyldithiodipropionamide
[0052] Raw material addition is identical with embodiment 1.
[0053] Add the N,N-dimethylformamide solution of methylamine dropwise to dimethyl disulfide dipropionate, the dropping temperature is controlled at 0-5°C, and after the dropwise addition, keep the reaction at 20-25°C for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com