Gene engineering bacterium, preparation and use thereof
A genetically engineered bacteria and gene technology, applied in the field of bioengineering, can solve the problems of protein without enzymatic activity, type I diabetes immune activity, unrealistic human GAD65 protein, and low expression of human GAD65, so as to avoid high price and production cost The effect of low cost and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
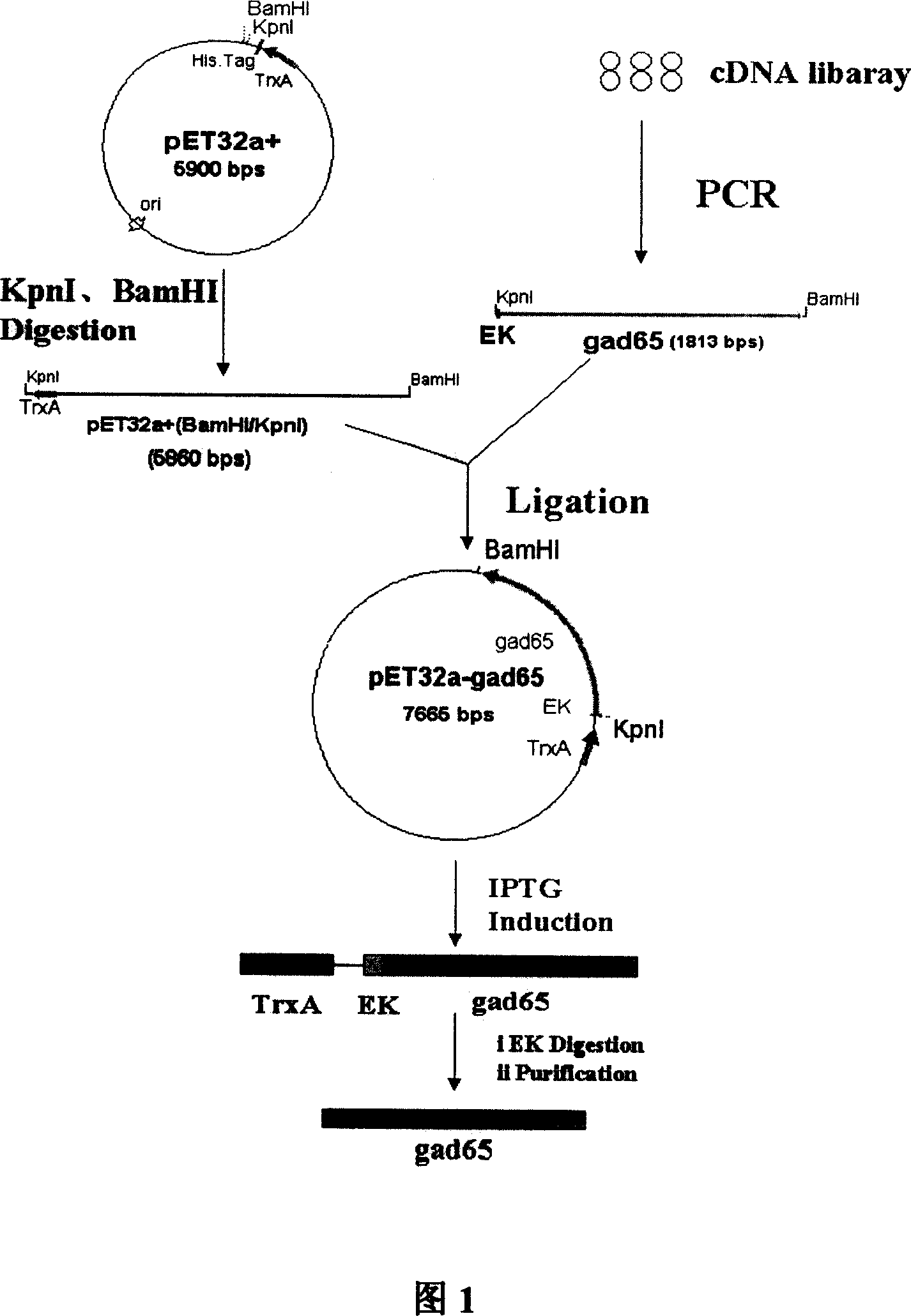
[0049] Embodiment 1: the preparation of genetically engineered bacteria
[0050] Step 1 Primer Design
[0051] According to the known cDNA sequence of human GAD65, specific Nest-PCR upstream primers A1, A2 and downstream primer B1 were designed, wherein KpnI restriction endonuclease and enterokinase cleavage sites were introduced into the upstream primer A2, and downstream A BamHI restriction endonuclease cutting site was introduced into primer B1.
[0052] Upstream primer A1:
[0053] 5'-GCGACCTGCTCCAGTCTCCAAAAG-3'
[0054] Upstream primer A2:
[0055] 5'-TTA GGTACCGACGACGACGACAAG GCATCTCCGGGCTCT-3'
[0056] KpnI enterokinase cleavage site
[0057] Downstream primer B1:
[0058] 5'-GCT GGATCC TGGAACAGCTTGGTGAGCA-3'
[0059] BamHI
[0060] The second step Nest-PCR amplification of human GAD65DNA fragment
[0061] Using the human pancreas cDNA library (purchased from Clontech) as a template, high-fidelity Pfu DNA polymerase was used to amplify GAD65,...
Embodiment 2
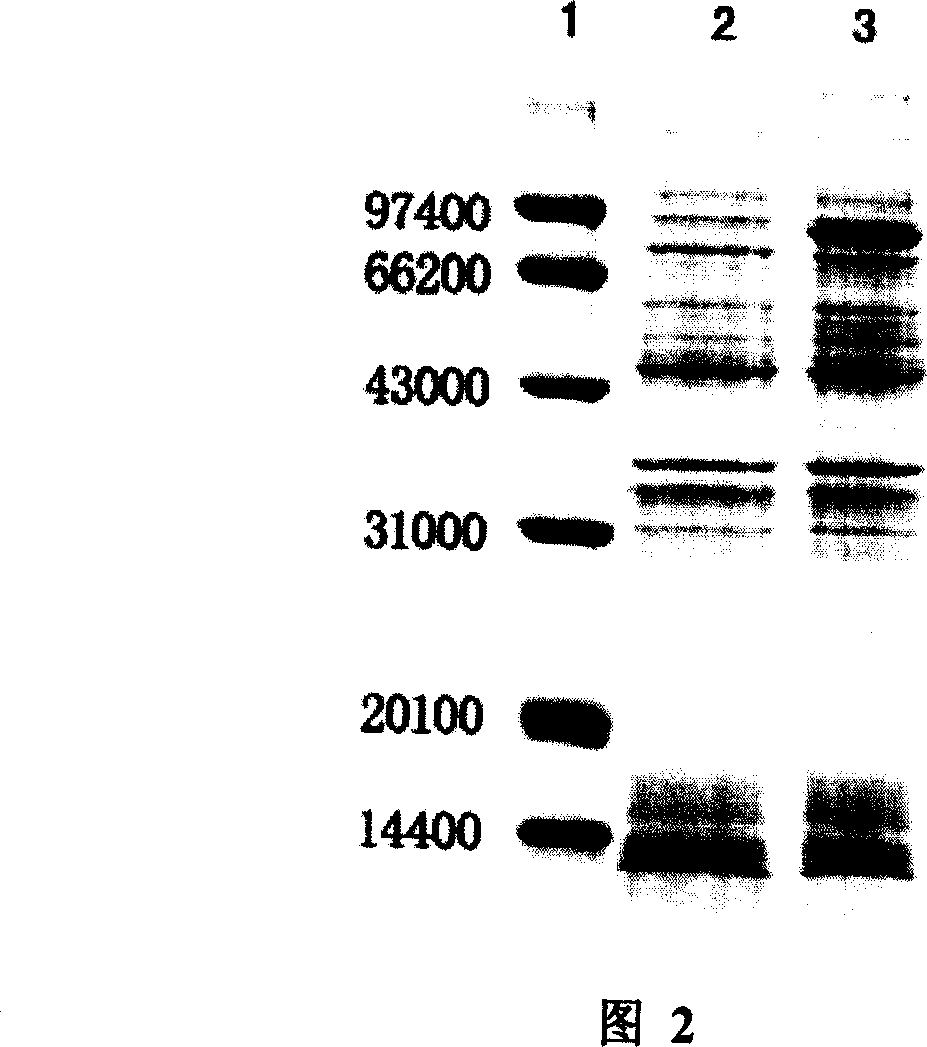
[0066] Example 2 Preparation of Thioredoxin-Human Glutamic Acid Decarboxylase 65 Fusion Protein Using the Genetically Engineered Bacteria of the Present Invention
[0067] An application of a genetically engineered bacterium highly expressing human glutamic acid decarboxylase 65, that is, using the genetically engineered bacterium to produce human glutamic acid decarboxylase 65. The following affinity chromatography medium is Ni-NTA His.Bind or His.Bind resin, purchased from Novagen, and the following enterokinase is purchased from MERCK.
[0068] The first step liquid culture
[0069] The genetically engineered bacteria constructed in Example 1 were inoculated into 20 mL of LB liquid medium containing 100 mg / mL Amp, cultivated at 37°C, 210rpm to OD600=0.6-1.0. Centrifuged at 5000rpm, 4°C for 5min, and collected the precipitated Bacteria were stored overnight at 4°C. The next day, 20 mL of LB medium containing 100 mg / mL Amp was used to resuspend the bacteria, and 4% volume w...
Embodiment 3
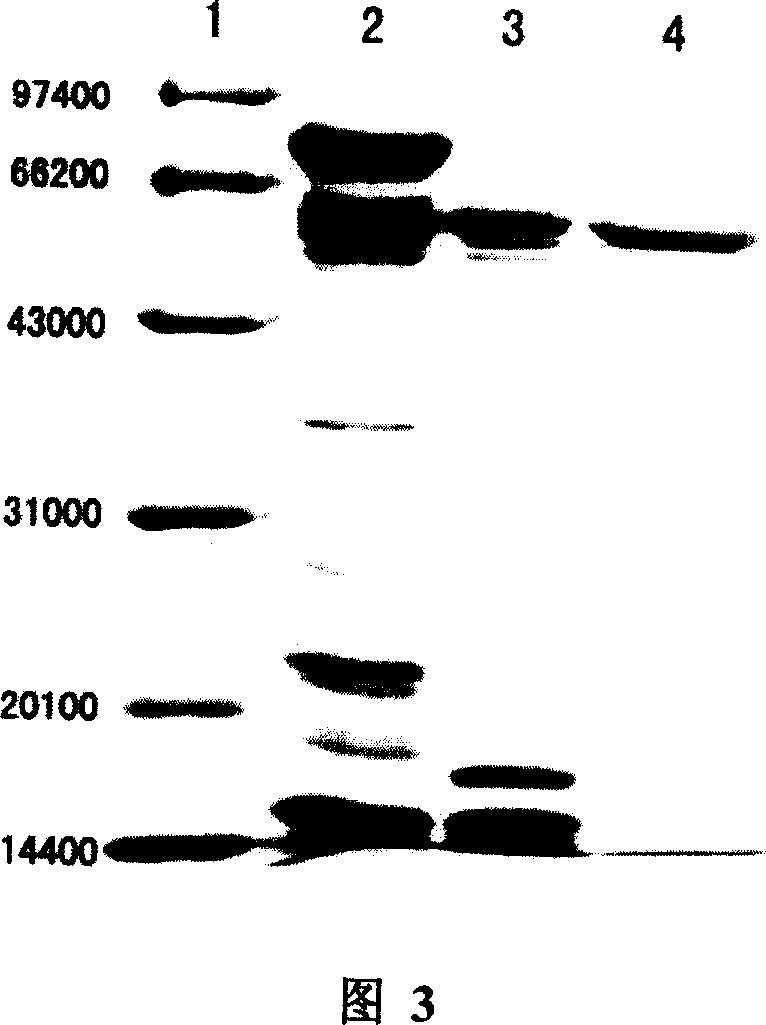
[0072] Embodiment 3 Utilizes the genetically engineered bacteria of the present invention to prepare human glutamic acid decarboxylase 65
[0073] Human glutamic acid decarboxylase 65 was prepared on the basis of the second step in Example 3. The fusion protein TrxA-GAD65 was digested with enterokinase. Add 1 unit of enterokinase per mg of fusion protein, shake at 25°C, and digest for about 16 hours. Digested products were separated by affinity chromatography Ni-NTA His.Bind or His.Bind resin. The breakthrough peak and the elution peak of NTA / IDA-0 were collected. The two fractions were combined and GAD65 was desalted and concentrated using 5KD-10KD MilliporeAmicon Ultra-15 ultrafiltration tubes. Protein concentration was determined by the Lowry method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com