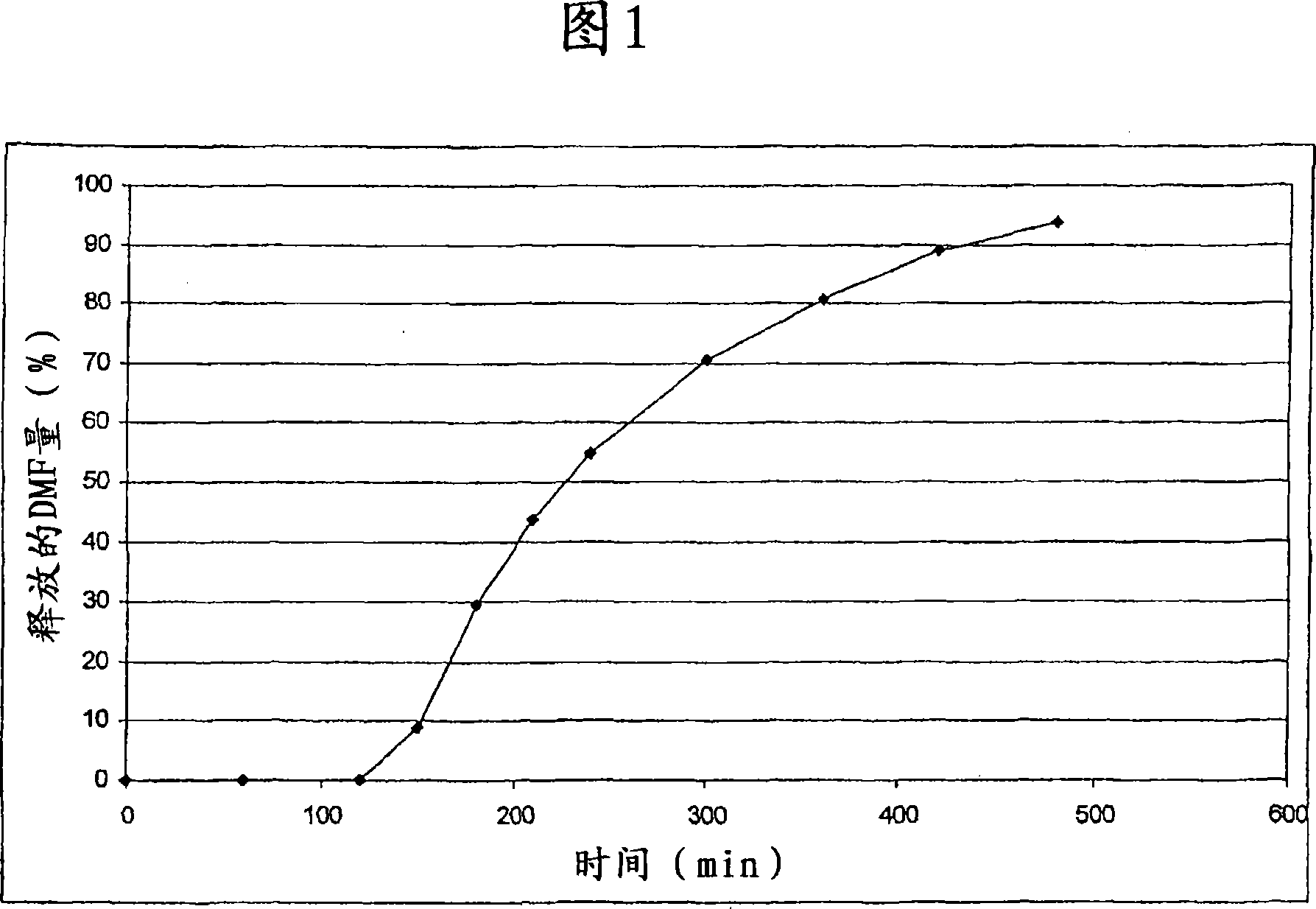
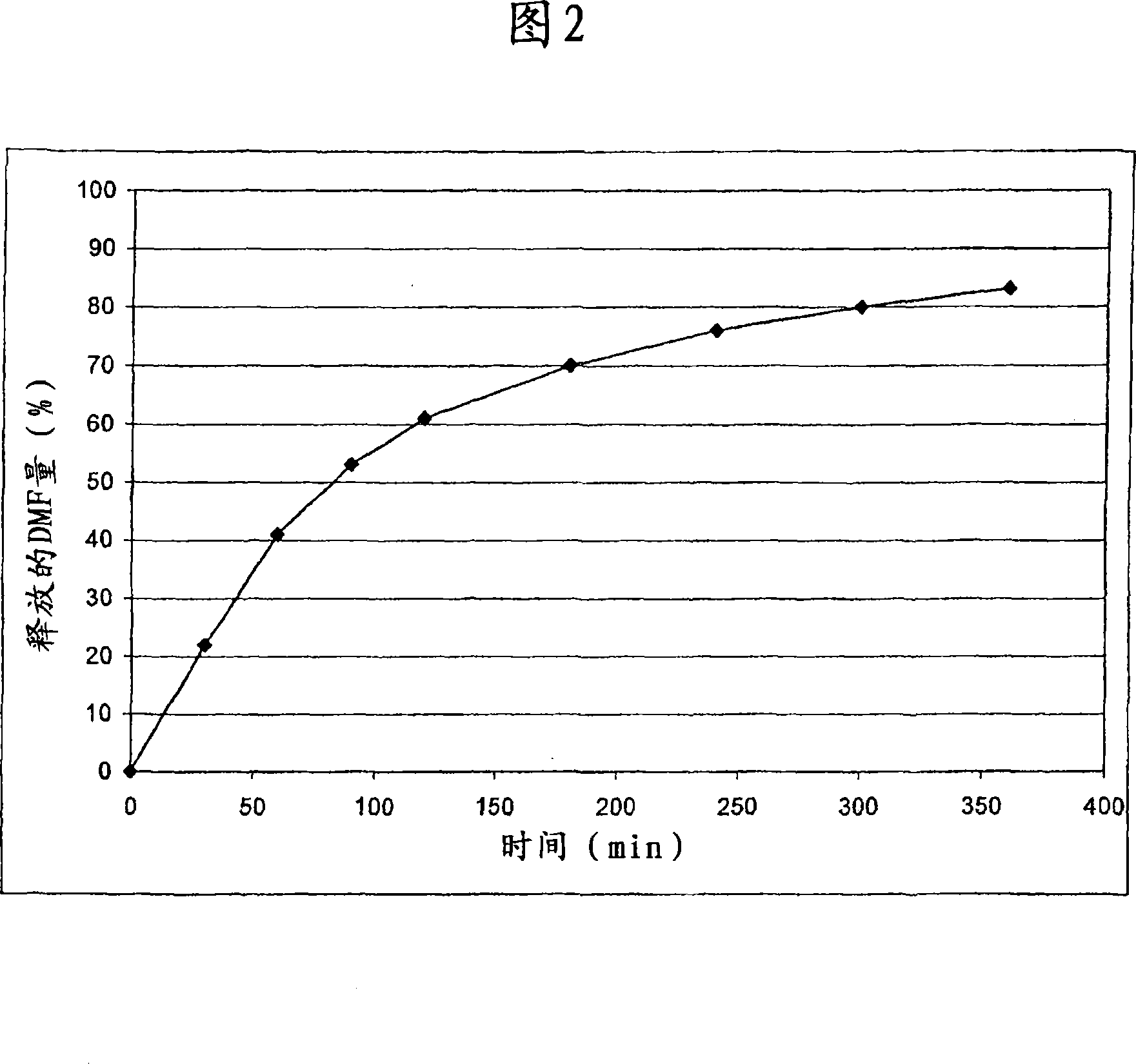
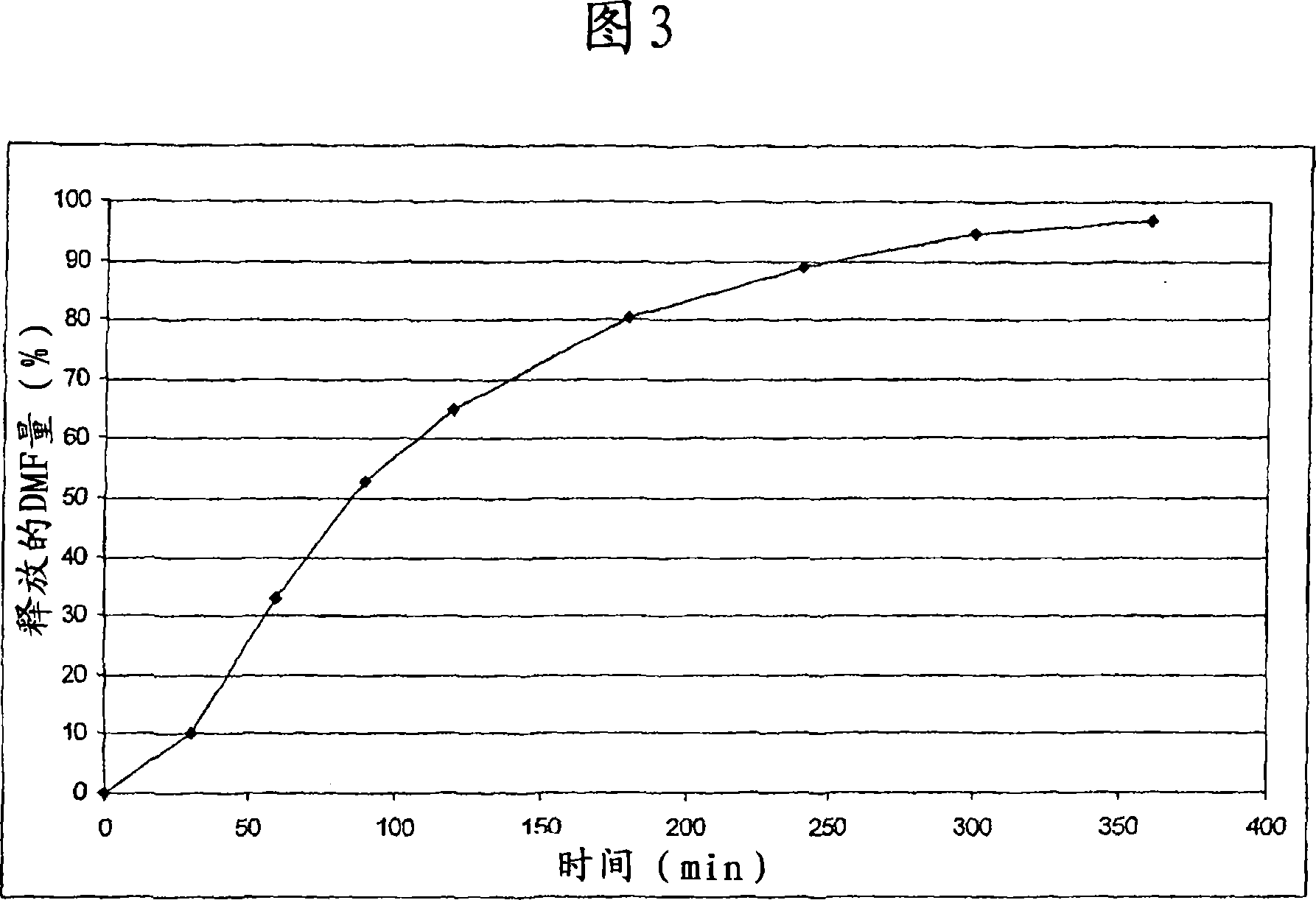
Controlled release pharmaceutical compositions comprising a fumaric acid ester
A technology of fumarate and controlled release drugs, which is applied in the direction of pill delivery, etc., and can solve problems such as gastrointestinal side effects and colic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0294] Tablet preparation
[0295] Mix 200 g of granules with 150 g of microcrystalline cellulose (such as Avicel 102), 97.5g lactose (such as Tablettose ), 10g sodium carboxymethylcellulose (such as Ac-Di-Sol ) and 25g of starch were mixed for 30min. Then 10 g of magnesium stearate and 7.5 g of amorphous silicon dioxide (such as Aerosil 200), the powder mixture was mixed for 5 min.
[0296] This powder mixture is compressed into tablets in tableting equipment (tablet diameter 10mm, surface area approximately 280-300mm 2 ). Enteric coating was applied during pan coating or fluidized bed coating, as described in Example 4.
Embodiment 2
[0298] Tablet preparation
[0299] Mix 200g microcrystalline with 150g microcrystalline cellulose (such as Avicel 102), 130g lactose (e.g. Tablettose ), 10g sodium carboxymethylcellulose (such as Ac-Di-Sol ) and 25mg of starch were mixed for 30min. Then 10 g of magnesium stearate and 7.5 g of amorphous silicon dioxide were added and the powder mixture was mixed for 5 min. This powder mixture is compressed into tablets in tableting equipment (tablet diameter 10mm, surface area approximately 280-300mm 2 ). Enteric coating was applied during pan coating or fluidized bed coating, as described in Example 4.
Embodiment 3
[0301] Preparation of capsules
[0302] The granules or microcrystals were filled into HPMC capsules and enteric coated as described below. In a pan coater, spray the capsules with Eudragit L30D-55, drying temperature 60°C to 80°C, dosage 20mg polymeric material per mm 2 . Add appropriate amount of coloring and talc.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com