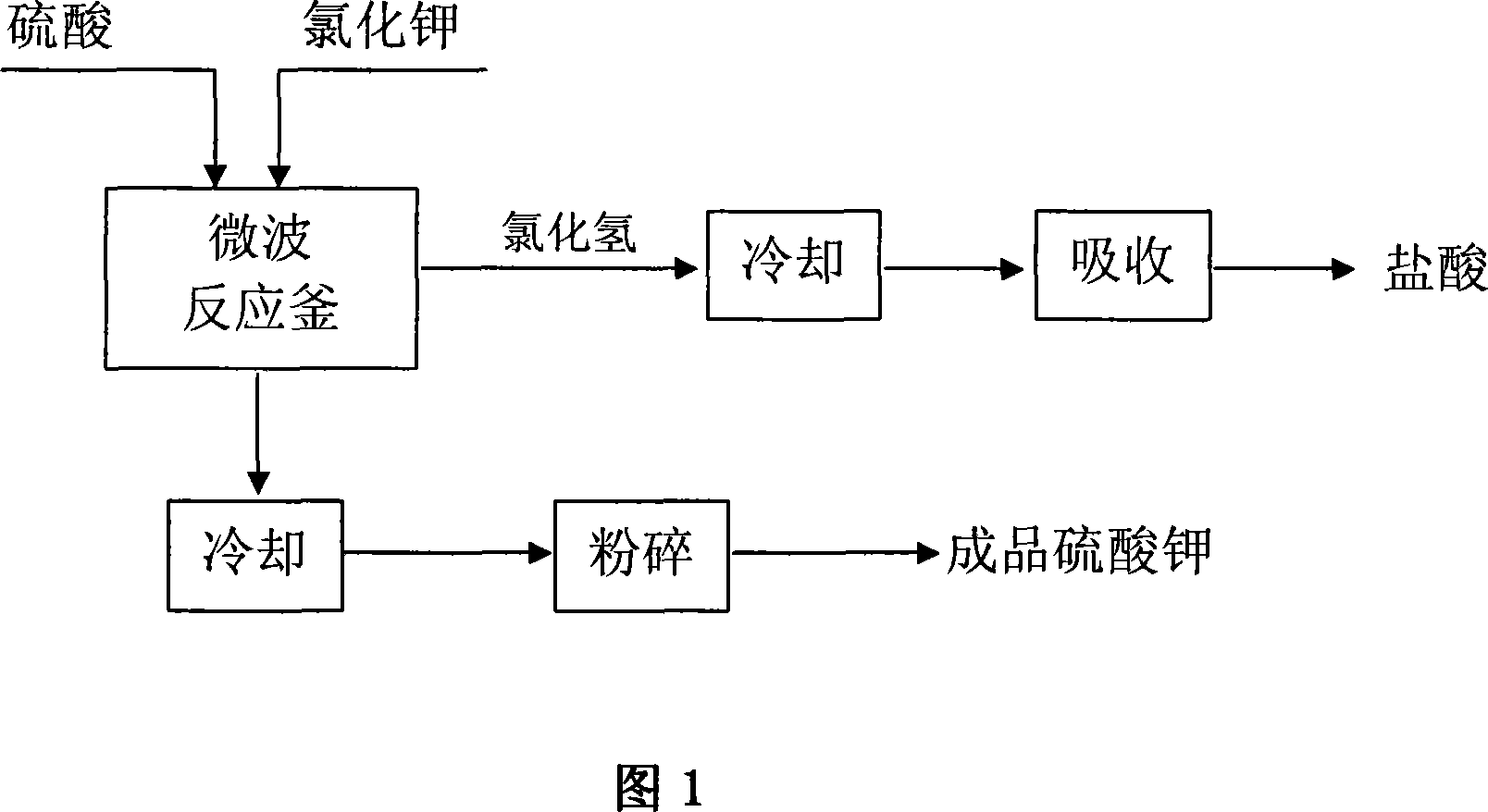
Microwave radiation process for producing potassium sulfate
A technology of microwave radiation and potassium sulfate is applied in the field of inorganic salt industry and chemical fertilizer industry to achieve the effect of accelerating the reaction speed and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Potassium chloride with a content of 95% and industrial sulfuric acid with a concentration of 98% are dropped into a microwave reactor at a ratio of 0.85:0.57 by weight, and reacted with 2450MHz microwave radiation under continuous stirring, and the reaction temperature is controlled at 400°C. Radiation time is about 50 minutes. After the reaction is completed, the solid product is cooled and pulverized to obtain the finished potassium sulfate, and the hydrogen chloride gas generated by the reaction is cooled and then absorbed with water to obtain a hydrochloric acid by-product with a concentration of 31%.
Embodiment 2
[0013] Put potassium chloride with a content of 95% and industrial sulfuric acid with a concentration of 93% into a microwave reactor in a ratio of 0.85:0.60 by weight, under continuous stirring, react with 4450MHz microwave radiation, and the reaction temperature is controlled at 800°C. The radiation time is about 40 minutes. After the reaction is completed, the solid product is cooled and pulverized to obtain the finished potassium sulfate. The hydrogen chloride gas generated by the reaction was cooled and absorbed with water to obtain a hydrochloric acid by-product with a concentration of 31%.
Embodiment 3
[0015] The potassium chloride with a content of 95% and the industrial sulfuric acid with a concentration of 98% are dropped into a microwave reactor in a ratio of 0.90:0.60 by weight, and reacted with 5000MHz microwave radiation under continuous stirring, and the reaction temperature is controlled at 700°C. Radiation time is about 45 minutes. After the reaction is completed, the solid product is cooled and pulverized to obtain the finished potassium sulfate. The hydrogen chloride gas generated by the reaction was cooled and absorbed with water to obtain a hydrochloric acid by-product with a concentration of 31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com