C15phosphonate ester compound, preparation method application thereof
A carbon pentadecanephosphonate and compound technology, applied in the field of 3-methyl-5--2, canthaxanthin synthesis, can solve the problems of low reaction yield, strong corrosiveness, difficult preparation and the like, and achieves the reaction yield. High rate, low cost, and the effect of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
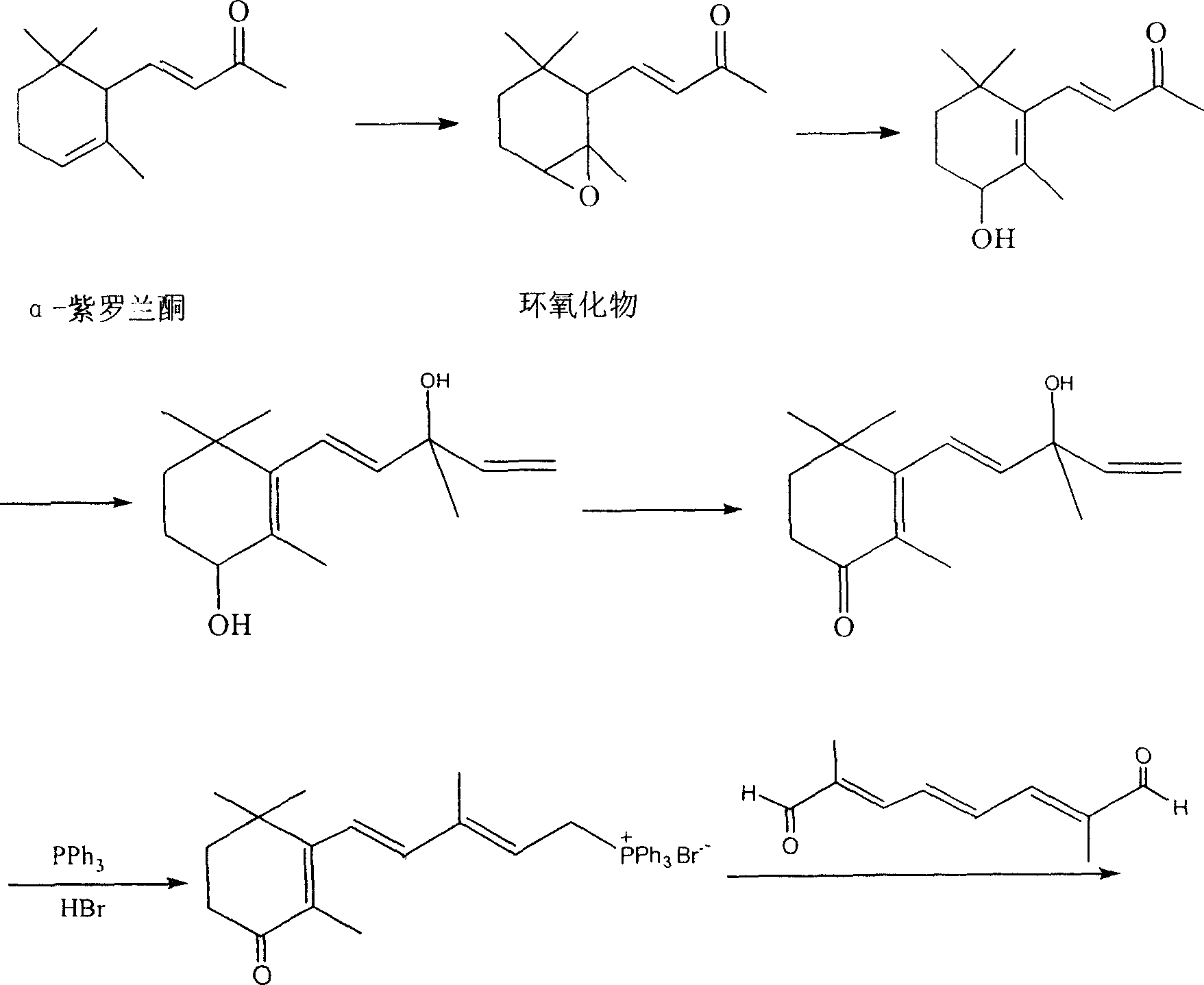
[0022] Example 1: Synthesis of 2-methyl-4-(2,6,6-trimethyl-3-hydroxycyclohexen-1-yl)-2-butenal
[0023] Mix 40g of 4-(2,6,6-trimethyl-3-hydroxy-1-cyclohexen-1-yl)-3-buten-2-one with 40g of methyl chloroacetate and cool to below -10°C , the temperature was controlled below -10°C, and 250 g of 30% sodium methoxide in methanol was added dropwise. Add 100mL of water, neutralize with acetic acid to neutrality, then add 200mL of ethyl acetate, separate the layers, then wash the layers with 100mL saturated aqueous sodium chloride solution, and wash the layers with 100 mL of saturated sodium chloride solution. Dry over magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain 35 g of 2-methyl-4-(2,6,6-trimethyl-3-hydroxycyclohexen-1-yl)-2-butenal , yield 82%, GC content 91%. Boiling point: 105-108°C (2mmHg). 1 HNMR (400Hz, CDCl3): 0.990(s, 3H, CH3), 1.042(s, 3H, CH3), 1.681(s, 6H, CH3), 1.816(s, 4H, CH2 CH2), 2.180(s, 1H, OH), 3.051~3.064(d, 2H, CH2), 3...
Embodiment 2
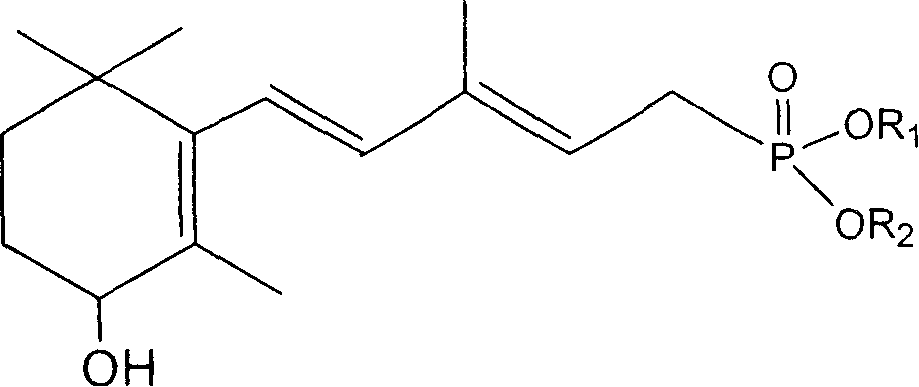
[0024] Example 2: Synthesis of 3-methyl-5-(2,6,6-trimethyl-3-hydroxycyclohexen-1-yl)-1,3-pentadienephosphonic acid diethyl ester
[0025] Weigh 6.5g of 60% sodium hydride, wash with n-hexane, add 150mL of anhydrous toluene, add dropwise a solution of 50g of diethyl methylene bisphosphonate in 20mL of anhydrous toluene under nitrogen protection, and control the temperature at 25°C Next, after the dropwise addition was completed and then reacted for half an hour, 35g of 2-methyl-4-(2,6,6-trimethyl-3-hydroxycyclohexen-1-yl)-2-butenal was dissolved in 50mL The solution of anhydrous toluene was added dropwise at a temperature below 25°C. After the dropwise addition, the reaction was continued for one hour. After the reaction was completed, 200 mL of water was added to wash and separate the layers. The organic layer was dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain 3-formazol 49 g of diethyl-5-(2,6,6-trimethyl-3-hyd...
Embodiment 3
[0027] Example 3: Synthesis of 3-methyl-5-(2,6,6-trimethyl-3-hydroxycyclohexen-1-yl)-2,4-pentadienephosphonic acid diethyl ester
[0028]Weigh 4.0 g of potassium tert-butoxide, dissolve it in 150 mL of anhydrous DMSO solution, add 3-methyl-5-(2,6,6-trimethyl-3-hydroxycyclohexene-1- Diethyl)-1,3-pentadienephosphonic acid diethyl ester 49g, control temperature 15-25 ℃, react for 2 hours after completion of dropwise addition, adjust pH to neutral with 10% aqueous acetic acid solution after reaction, add 200mL water and 200mL ethyl acetate, layered, the organic layer was washed once with 200mL water, layered, dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain 3-methyl-5-(2,6,6-tri 46.6 g of diethyl methyl-3-hydroxycyclohexen-1-yl)-2,4-pentadienephosphonate as a viscous oil. Yield 95%. 1 HNMR (400Hz, DMSO): 0.857(s, 3H, CH3), 1.002(s, 3H, CH3), 1.252~1.330(6H, CH3), 1.260~1.362(m, 2H, CH2), 1.524~1.646(m, 2H, CH2) 1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com