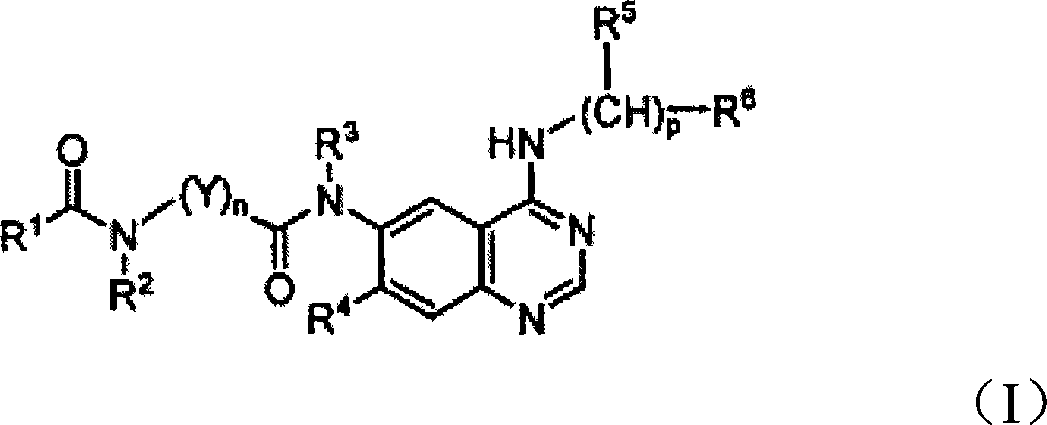
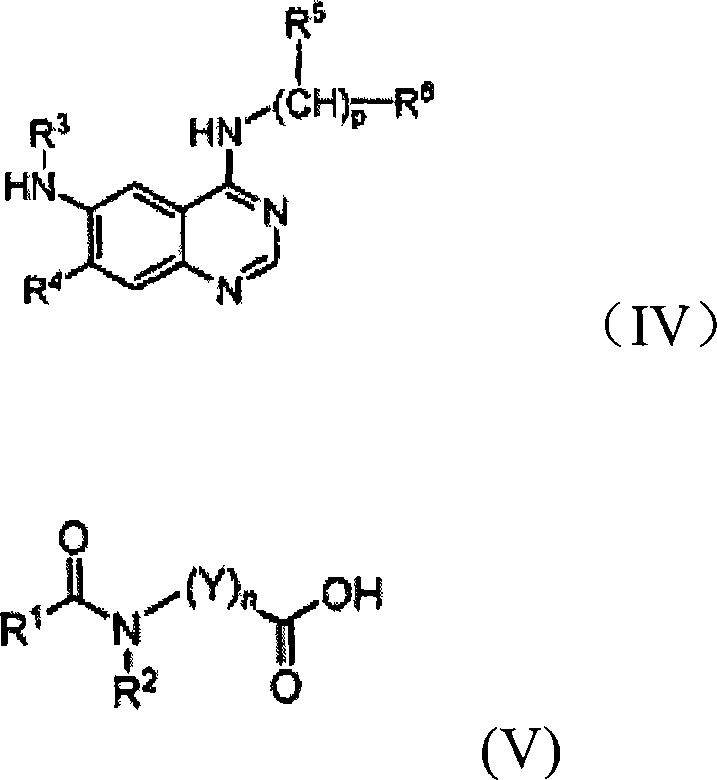
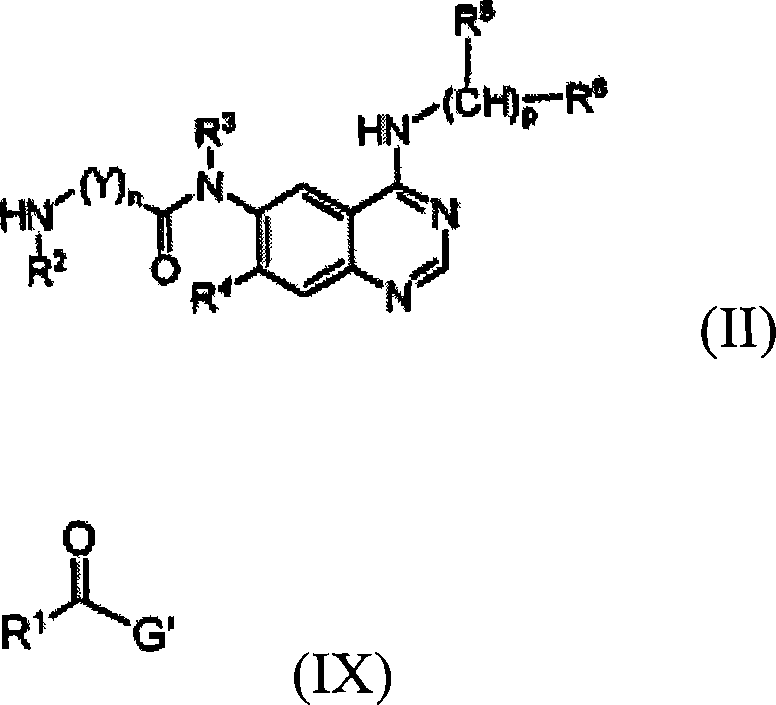
Quinazoline derivatives for inhibiting cancer cell growth and method for the preparation thereof
A kind of technology of quinazoline and derivatives, applied in the field of quinazoline derivatives and medicinal salts thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0339] Example 1 : Preparation of ({4-[3-chloro-4-(3-fluoro-benzyloxy)-phenylamino]-quinazolin-6-ylcarbamoyl}-methyl)-carbamic acid t-butyl ester
[0340] (1-1) Preparation of 6-nitro-3H-quinazolin-4-one
[0341] 150 g of 2-amino-5-nitro-benzoylic acid was added to 200 ml of formamide, and the resulting solution was heated to 170°C for 4 hours, and cooled to 100°C. 500 ml of ice water was added thereto, stirred for 1 hour, and the resulting mixture was filtered under reduced pressure. The obtained residue was washed with water to obtain a solid, which was dried at 40°C for 15 hours to obtain the title compound of the above formula (140 g, 90%).
[0342] 1 H-NMR(DMSO-d 6 , 300MHz): δ8.77 (d, J = 2.7 Hz, 1H), 8.55 (dd, J = 6.9 Hz, 1H), 8.36 (s, 1H), 7.84 (d, J = 9 Hz, 1H).
[0343] (1-2) Preparation of 4-chloro-6-nitro-quinazoline hydrochloride
[0344] Add 415ml of thionyl(di)chloride and 123ml of phosphorousoxy chloride to 80g of the compound obtained in (1-1), add 3ml of N,N-dim...
Embodiment 2
[0355] Example 2 : Preparation of N-({4-[3-chloro-4-(3-fluoro-benzyloxy)-phenylamino]-quinazolin-6-ylcarbamoyl}-methyl)-2-methoxy Base-acetamide
[0356] (2-1) Preparation of (2-Methoxy-Acetylamino)-Ethyl Acetate
[0357] 1.5 ml of triethylamine was added to 500 mg of amino-ethyl acetate hydrochloride dissolved in 5 ml of chloroform, and the solution was cooled to -78°C. 0.34 ml of methoxyacetyl chloride diluted with 3 ml of chloroform was slowly added thereto, and the resulting solution was slowly heated to room temperature and reacted for 6 hours. The reacted solution was washed with distilled water, adjusted to pH 8-9 by adding saturated sodium bicarbonate solution, extracted with an organic solvent, dried over magnesium sulfate, filtered and distilled under reduced pressure to obtain the title compound (595 mg, 75% ).
[0358] 1 H-NMR(CDCl 3 , 300MHz): δ7.15 (br s, 1H), 4.26 (q, 2H), 4.11 (d, 2H), 3.98 (s, 2H), 3.48 (s, 3H), 1.33 (t, 3H).
[0359] (2-2) Preparation of (2-methox...
Embodiment 3
[0368] Example 3 : Preparation of N-({4-[3-chloro-4-(3-fluoro-benzyloxy)-phenylamino]-quinazolin-6-ylcarbamoyl}-methyl)-2-methanesulfon Acyl-acetamide
[0369] Add 16 mg of 1-hydroxybenzotriazole and 53 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to 18 mg of methanesulfonyl acetic acid dissolved in 3 ml of THF. After 50 mg of the compound obtained in Example 2 (2-1), the solution was reacted for 2 hours. The reaction solution was extracted with distilled water, and the obtained residue was filtered under reduced pressure to obtain the title compound (44 mg, 70%).
[0370] 1 H-NMR(CDCl 3 , 300MHz): δ 8.60 (s, 1H), 8.52 (s, 1H), 7.82 (d, 1H), 7.69 (m, 2H), 7.54 (dd, 1H), 7.31 (m, 2H), 7.21 (t , 2H), 6.97(m, 2H), 5.12(s, 2H), 4.12(s, 2H), 4.03(s, 2H), 3.14(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com