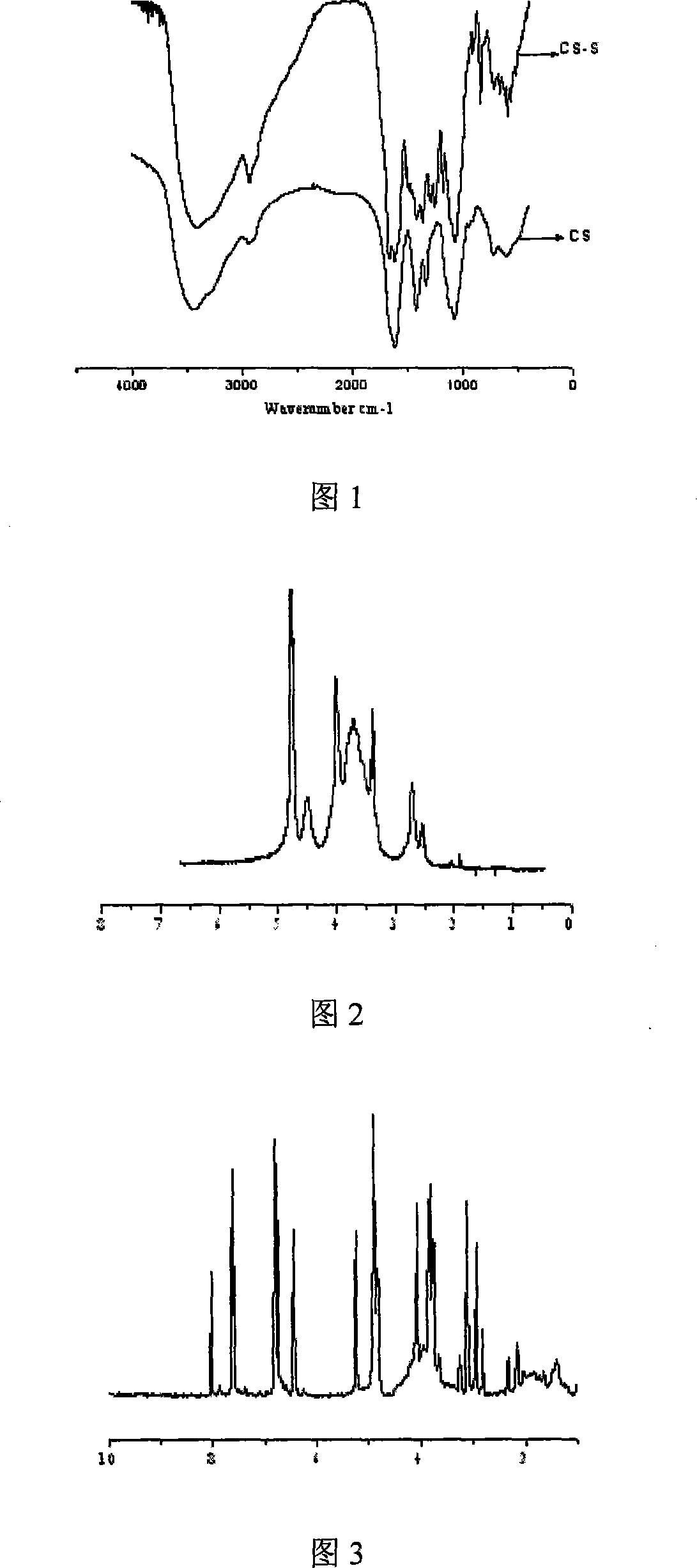
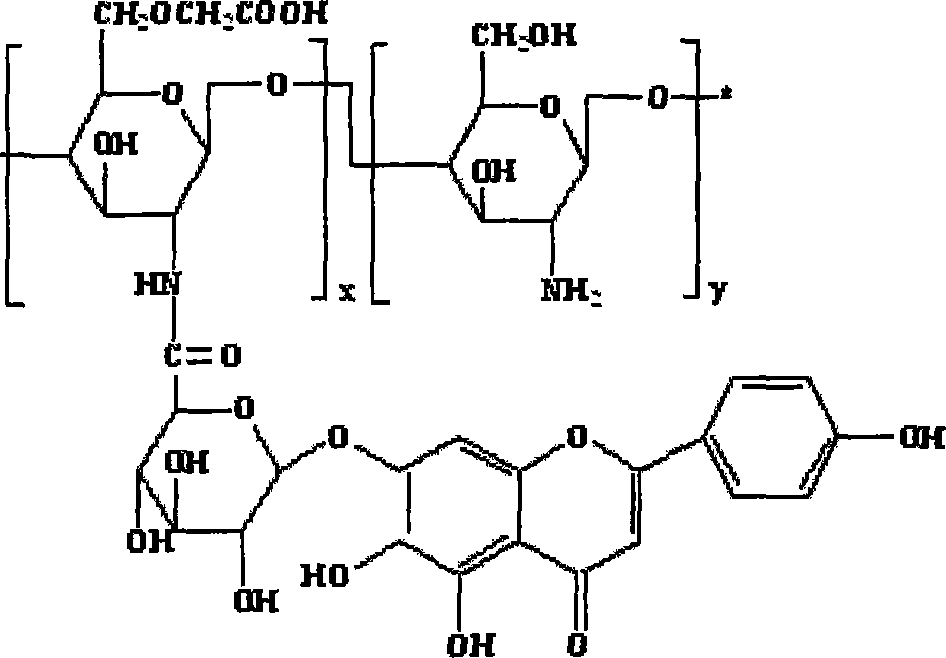
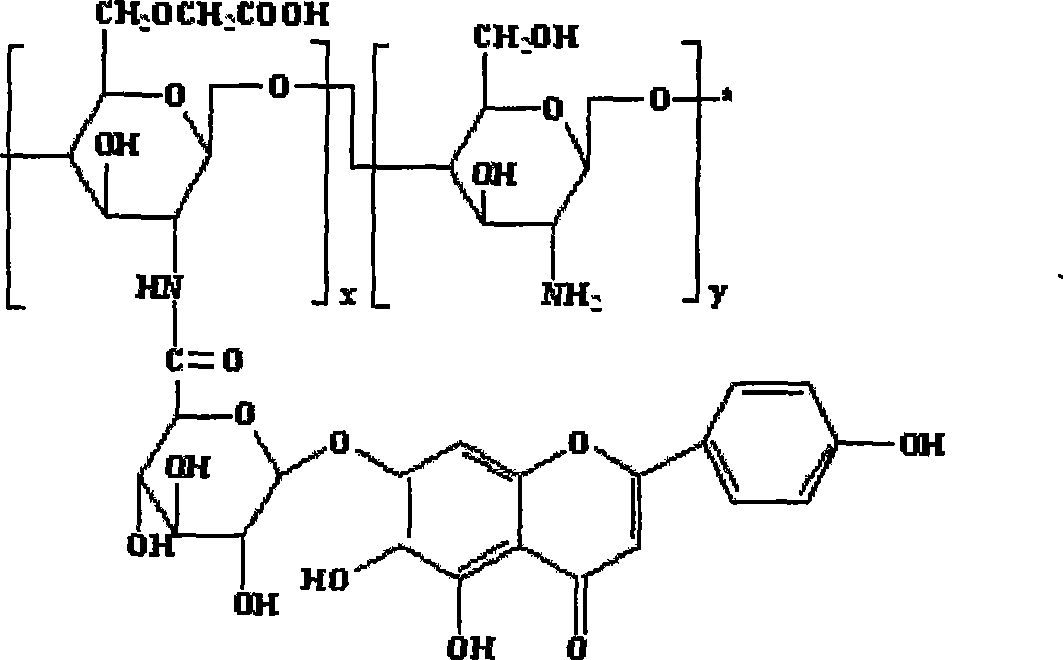
Scutellarin prodrug using carboxymethyl chitosan as the carrier and method for preparing the same
A technology of carboxymethyl chitosan and scutellarin, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of ethylene glycol and other problems, and achieve easy realization , improve bioavailability, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Carboxymethyl chitosan (0.35 g, 1.5 mmol) prepared from methyl chitosan with a molecular weight of 2000 was dissolved in 100 ml of distilled water and stirred to fully dissolve it. Dissolve scutellarin (0.70 g, 1.5 mmol) in 20 ml of DMF, and add dropwise to the carboxymethyl chitosan solution with a dropping funnel. The above reaction solution was fully stirred, and the solution became light yellow, transparent and clear, and then 0.15 g of DMAP was added. DCC (0.35 g, 1.5 mmol) was dissolved in 10 ml of DMF, dropped into the reaction solution with a dropping funnel, and stirred at 50° C. for 24 hours. After the reaction is finished, filter the filtrate with a slow filter paper, centrifuge the filtrate, put the clear liquid in a pear-shaped bottle, and evaporate to dryness. Wash the light green solid on the wall of the pear-shaped bottle with n-propanol to form a large amount of precipitate, filter it, wash it with n-propanol until the filtrate is colorless, dry the pr...
Embodiment 2
[0037] Carboxymethyl chitosan (0.24 g, 1.0 mmol) prepared from methyl chitosan with a molecular weight of 4000 was dissolved in 80 ml of distilled water and stirred to fully dissolve. Dissolve scutellarin (0.94g, 2.0mmol) in 30ml DMF, and add it dropwise to the carboxymethyl chitosan solution with a dropping funnel. The above mixed solution was stirred sufficiently, and the solution became light yellow, transparent and clear, and then 0.10 g of DMAP was added. DCC (0.24 g, 1.0 mmol) was dissolved in 10 ml of DMF, and dropped into the above mixture with a dropping funnel. The reaction was stirred at 40°C. 120 hours. After the reaction is finished, filter with slow filter paper and centrifuge the filtrate, take the clear part and place it in a pear-shaped bottle, and evaporate to dryness. Wash the light green solid on the wall of the pear-shaped bottle with n-propanol to form a large amount of precipitate, filter it, wash it with n-propanol until the filtrate is colorless, dr...
Embodiment 3
[0039] Carboxymethyl chitosan (1.05 g, 4.5 mmol) prepared from methyl chitosan with a molecular weight of 20,000 was dissolved in 80 ml of distilled water and stirred to fully dissolve. Dissolve scutellarin (2.33g, 5.0mmol) in 30ml DMF, and add it dropwise to the carboxymethyl chitosan solution with a dropping funnel. The above mixed solution was stirred sufficiently, and the solution became light yellow, transparent and clear, and then 0.50 g of DMAP was added. EDC (1.14g, 4.5mmol) was dissolved in 10ml of distilled water, and dropped into the above mixture with a dropping funnel. The reaction was stirred at 25°C. After 192 hours, filter with slow filter paper and centrifuge the filtrate, take the clear part and place it in a pear-shaped bottle, and evaporate to dryness. Wash the light green solid on the wall of the pear-shaped bottle with n-propanol to form a large amount of precipitate, filter it, wash it with n-propanol until the filtrate is colorless, and dry the precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com