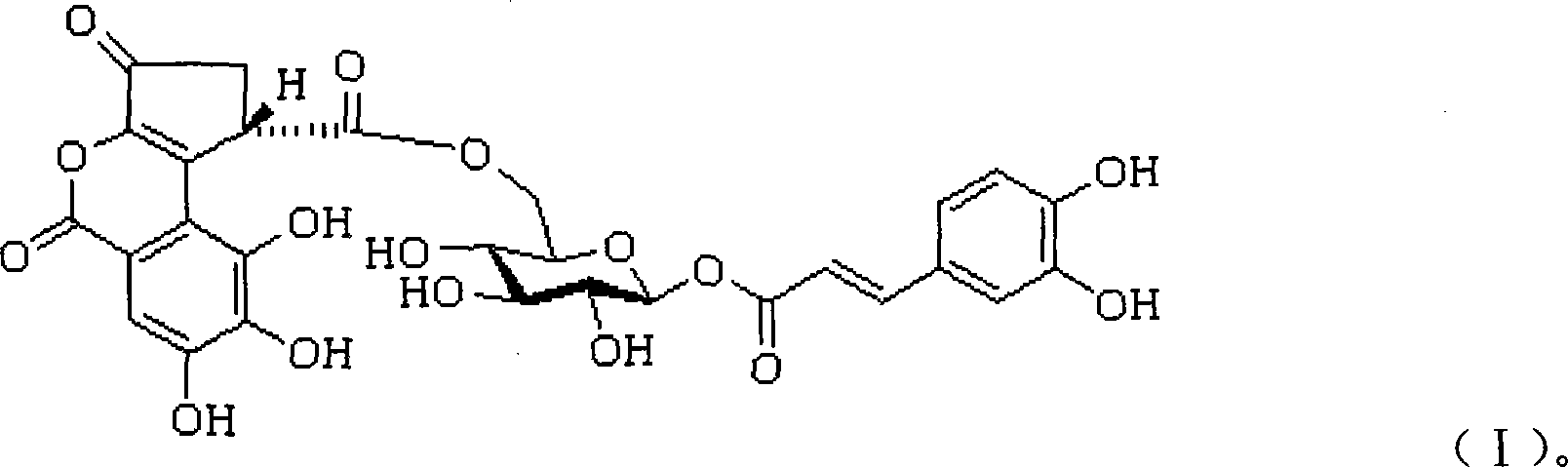
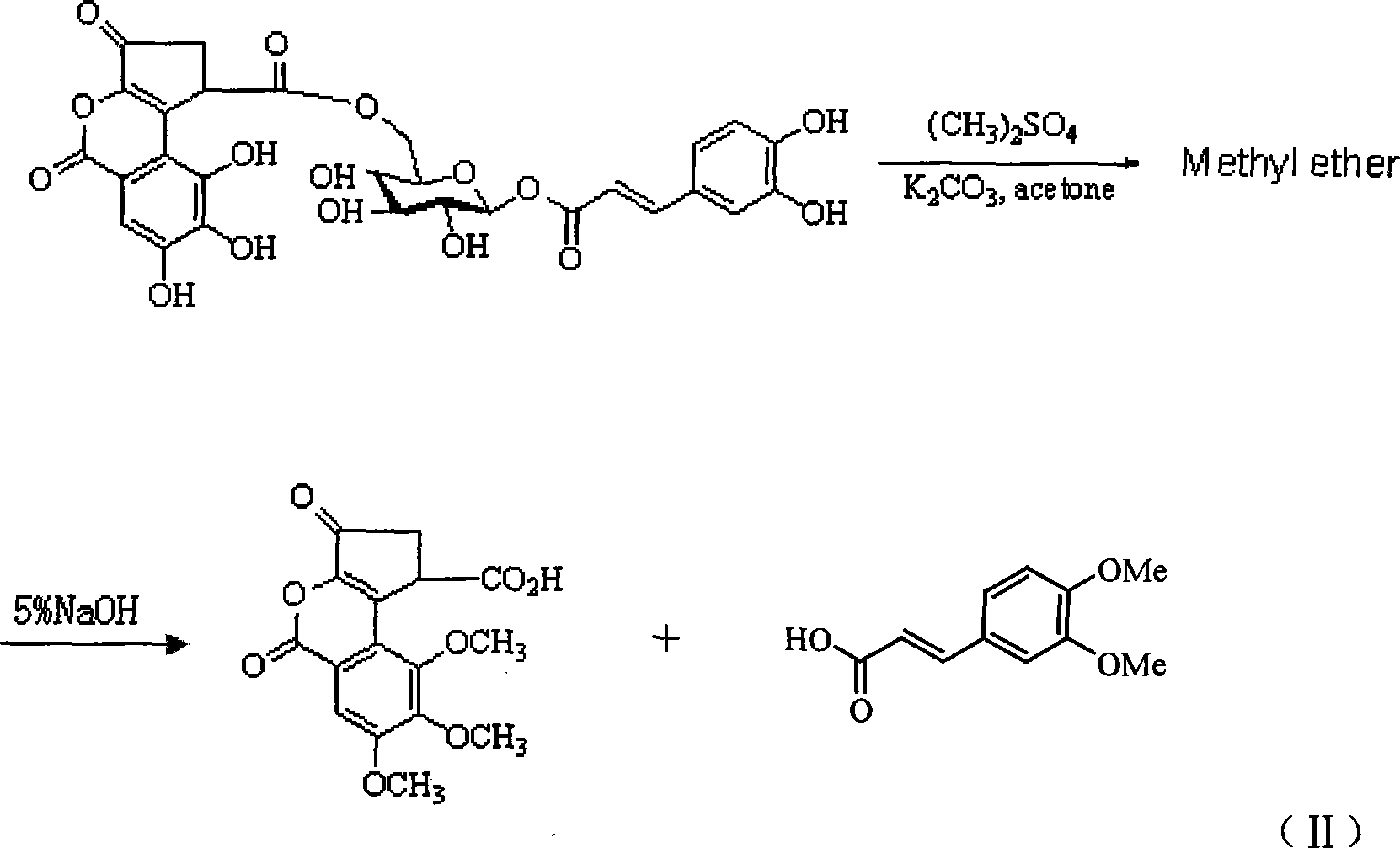
1-0-caffeoyl -6-0-(S)-caesalpinia sepiaria acyl group -Beta-D-glucopyranose and uses thereof
A technology of glucopyranose and caffeoyl, applied in the direction of sugar derivatives, sugar derivatives, esterified saccharides, etc., can solve the problems such as the use of such compounds is not disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0041]1. Take the snake wild rice produced in Japan, chop it, soak it with 70% methanol at room temperature for 24 hours, filter, and use a rotary evaporator to concentrate the filtrate to remove methanol, and the concentrated solution is the total extract. The methanol extract was extracted with ether and ethyl acetate respectively, and divided into three parts: ether layer, ethyl acetate layer and water layer. The water layer is the water-soluble part, which contains hydrolyzable tannins. The water-soluble part was subjected to MCI-gelCHP20P column chromatography, eluted with water and then eluted with 10%-40% methanol, and the methanol eluate was collected and passed through Chromatorex ODS, eluted with water and then eluted with a gradient of 10-80% methanol. Collect 40-80% methanol eluate, recover methanol under reduced pressure, add a little methanol to dissolve the residue; then apply MCI-gel CHP20P, elute with water and then gradient elution with 10-80% methanol, colle...
example 1
[0066] 【prescription】
[0067] 1-O-Caffeoyl-6-O-(S)-Ceremoyl-β-D-Glucopyranose 20g
[0068] Microcrystalline Cellulose 48g
[0069] Soluble starch 30g
[0071] A total of 1000 tablets were produced, each containing 20 mg of the compound.
[0072] 【Preparation】
[0073] Weigh 20g of the compound, add soluble starch to dilute to 50g, mix well, then weigh 48g of microcrystalline cellulose, use 95% ethanol to make soft material, sieve and granulate, dry at low temperature at 50°C, granulate, add talc powder 2g , mixed evenly, compressed into tablets (0.1g each), inspected for quality, packaged, and obtained.
[0074] 【Dosage】
[0075] 3 tablets each time, 3 times a day.
[0076] 【For people】
[0077] Applicable to various tumor patients.
example 2
[0079] 【prescription】
[0080] 1-O-Caffeoyl-6-O-(S)-Ceremoyl-β-D-Glucopyranose 10g
[0081] Microcrystalline Cellulose 48g
[0082] Soluble starch 40g
[0083] Talc powder 2g
[0084] A total of 1000 tablets were produced, each containing 10 mg of the compound.
[0085] 【Preparation】
[0086] Weigh 10g of the compound, add soluble starch to dilute to 50g, mix well, then weigh 48g of microcrystalline cellulose, use 95% ethanol to make soft material, sieve and granulate, dry at low temperature at 50°C, granulate, add talc powder 2g , mixed evenly, compressed into tablets (0.1g each), inspected for quality, packaged, and obtained.
[0087] 【Dosage】
[0088] 3 tablets each time, 3 times a day.
[0089] 【For people】
[0090] Applicable to various tumor patients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com