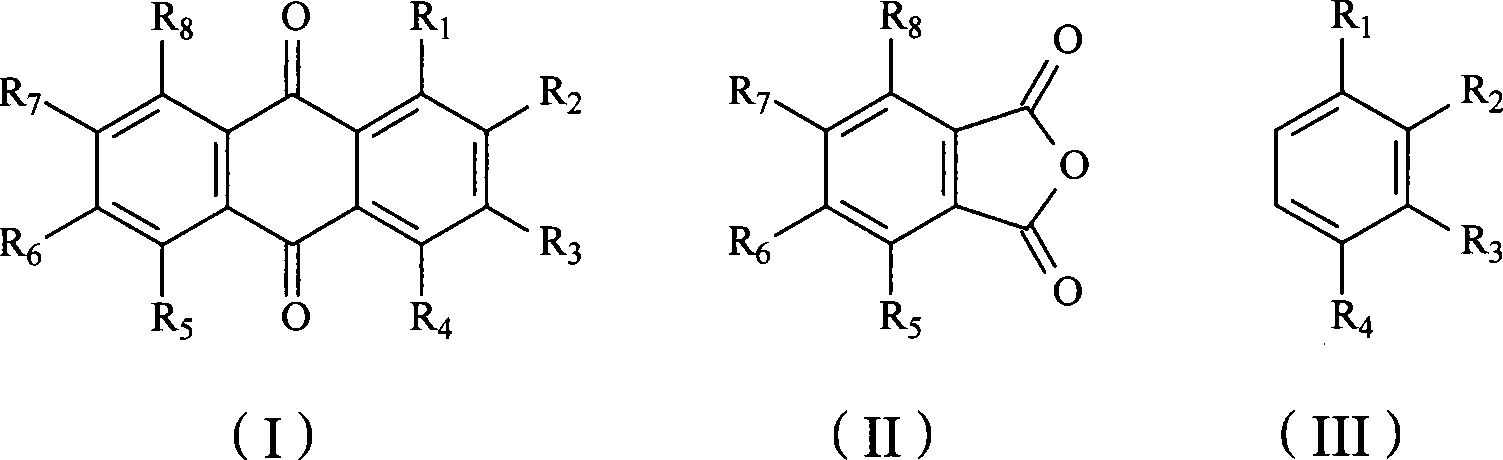
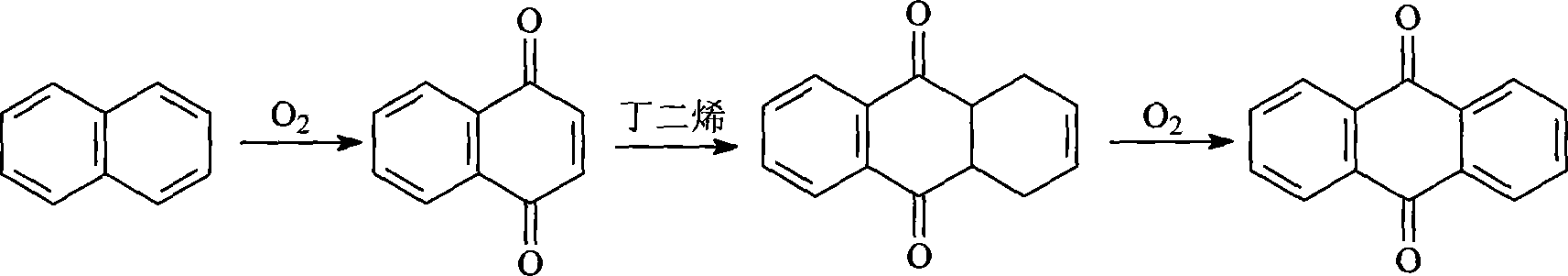
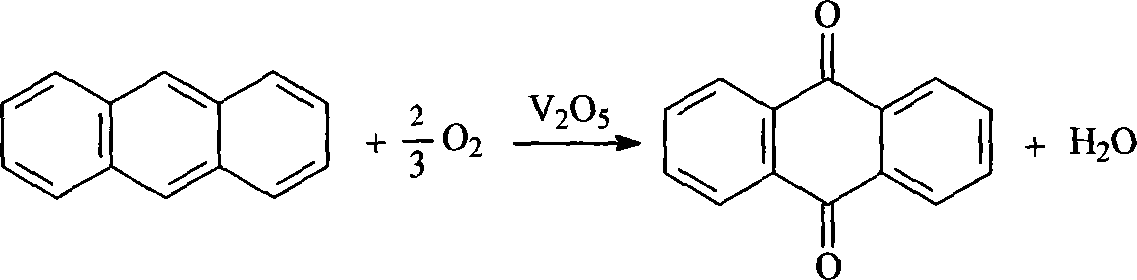Anthraquinone and preparation method for derivative thereof
A derivative, anthraquinone technology, applied in the field of preparation of anthraquinone and its derivatives, can solve the problems of three wastes, etc., and achieve the effects of low production cost, safe and reliable production, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 14.8g (0.1mol) of phthalic anhydride (phthalic anhydride), 11.0g (0.1mol) of hydroquinone and 0.25g of aluminum trichloride into the mortar, grind them thoroughly and put them into a 250mL dry flask, put them in Microwave oven, connect the condenser, set the power of the microwave oven to 650W, start the microwave oven, control the reaction temperature 300-350°C, and radiate for 5 minutes. After the reaction is completed, add 50mL of ice water, stir, suction filter, add soda ash to neutralize to pH=6.5 -7, add sodium hypochlorite solution (containing 3.0g of available chlorine), stir, and filter to obtain 22.4g of 1,4-dihydroxyanthraquinone, the yield is 91.5% (calculated as phthalic anhydride, the same below), and the purity is analyzed by high performance liquid chromatography was 98.1%.
Embodiment 2
[0031] The feed intake of hydroquinone is 13.2g (0.12mol), the microwave oven power is set to 1000W, and the radiation time is 1 minute. Other operations are the same as in Example 1 to obtain 23.3g of 1,4-dihydroxyanthraquinone, and the yield is 95.3%. , the purity was 98.3% by high performance liquid chromatography.
Embodiment 3
[0033] Add 77.4g of DMF (N,N-dimethylformamide) as a solvent, set the power of the microwave oven to 50W, control the reaction temperature to the reflux temperature (about 153°C), the input amount of the catalyst aluminum trichloride is 2.5g, microwave irradiation The time was 120 minutes, and other operations were the same as in Example 1 to obtain 23.5 g of 1,4-dihydroxyanthraquinone with a yield of 96.4% and a purity of 98.5% by high performance liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com