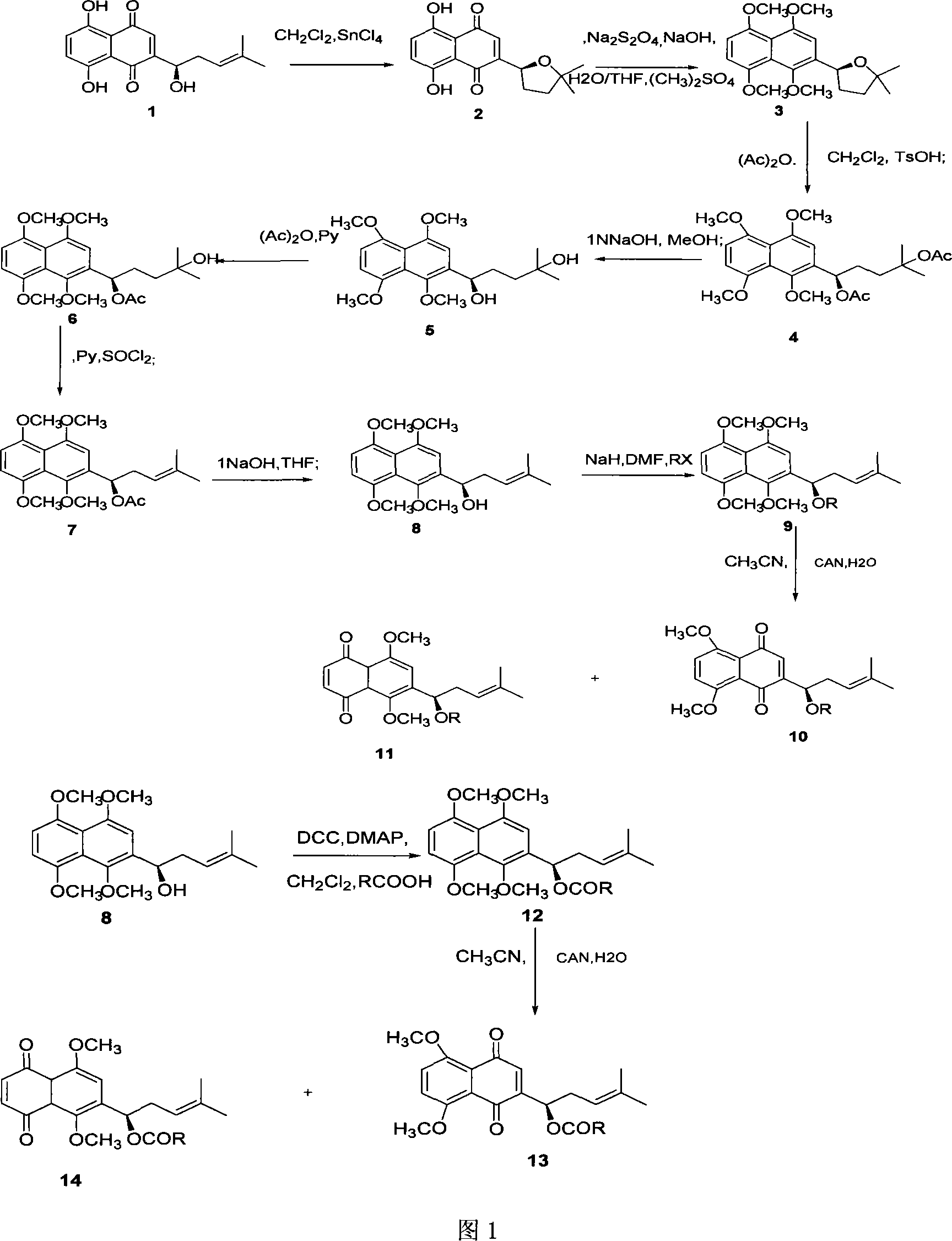
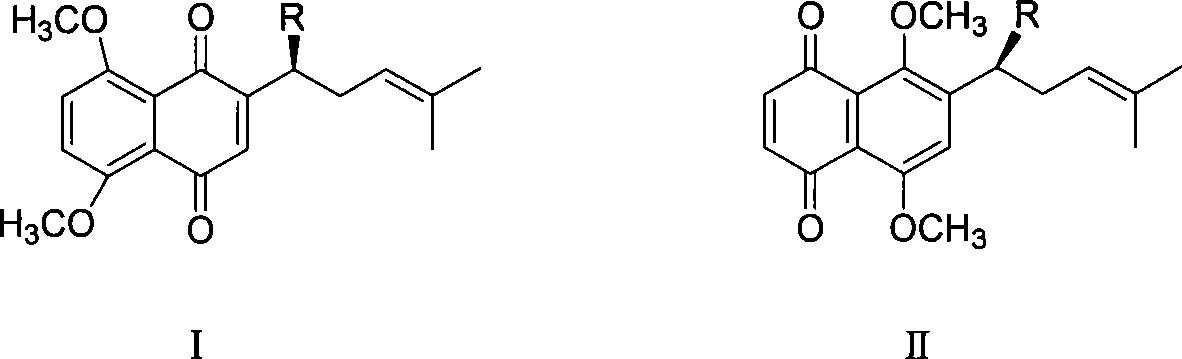
Method for synthesizing alkannin dimethyl ether derivative
A technology of shikonin dimethyl ether and synthesis method, which is applied in the field of synthesis of shikonin dimethyl ether derivatives and can solve problems such as incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] cyclized shikonin 2
[0025] Weigh 100 mg (0.347 mmol) of shikonin 1, dissolve it in 10 ml of anhydrous dichloromethane, and add 5 equivalents of tin tetrachloride. Under the protection of nitrogen, the reaction was carried out at room temperature for 0.5 hours. Add dichloromethane to dilute the reaction solution, wash with water and saturated brine several times, anhydrous MgSO 4 Dry, filter, and evaporate dichloromethane under reduced pressure. The crude product was chromatographed on a silica gel column, eluting with ethyl acetate:petroleum ether (1:15) to obtain 98.3 mg of the red target compound, with a yield of 98.3%. [α] D =+179.1° (C=0.01, CHCl 3 ), mp79-80℃, 1 H-NMR (300MHz, CDCl 3 ), δ (ppm): 12.53 (s, 1H, OH Ar ), 12.52 (s, 1H, OH Ar ), 7.23~7.19 (m, 3H, CH Ar ), 5.17 (dd, J=6.3, 5.7H Z , 1H, C H OH), 2.66-2.62 (m, 1H, CH 2 ), 1.90-1.89 (m, 1H, CH 2 ), 1.90~1.89 (m, 1H, CH 2 ), 1.88-1.74 (m, 1H, CH 2 ), 1.38 (s, 3H, CH 3 ), 1.35 (s, 3H, =CH 3...
Embodiment 2
[0027] tetraoxomethyl cyclized shikonin 3
[0028] Under nitrogen protection, 100 mg (0.347 mmol) of cyclized shikonin 2 was dissolved in 4 ml of water / tetrahydrofuran (1:4), and 5 times equivalents of sodium hydroxide, 10 equivalents of sodium hydroxide, and 10 equivalents of Dimethyl sulfate, stir well. After half an hour, reflux for 36 hours, cool to room temperature, add dichloromethane to the reaction solution, wash the dichloromethane layer with water and saturated brine, and anhydrous MgSO 4 After drying, dichloromethane was evaporated under reduced pressure. The resulting crude product was chromatographed on a silica gel column, eluting with ethyl acetate:petroleum ether (1:4) to obtain 95.6 mg of a light yellow oily compound, with a yield of 80.3%. 1 H-NMR (300MHz, CDCl 3 ), δ (ppm): 7.12 (s, 1H, CH Ar ), 6.80 (s, 2H, CH Ar ), 5.52 (m, 1H, C H OH), 3.99(s, 3H, -OCH 3 ), 3.95(s, 3H.-OCH 3 ), 3.93(s, 3H.-OCH 3 ), 3.75(s, 3H.-OCH 3 ), 2.54-2.48 (m, 1H, CH 2 ),...
Embodiment 3
[0030] 1,4,5,8-tetramethoxy-2-(1’,4’-diacetyl-4’-methyl-3’-pentane)-naphthalene 4
[0031] Under the protection of nitrogen, 100 mg (0.291 mmol) of tetraoxomethyl cyclized shikonin 3 was dissolved in 2 ml of dichloromethane, 2 ml of acetic anhydride and an equivalent p-toluenesulfonic acid monohydrate were added, and at -10°C, React for 24 hours. , dilute the reaction solution with ethyl acetate, wash with 5% aqueous sodium bicarbonate solution, water, and saturated brine, respectively, and evaporate the solvent under reduced pressure to obtain a crude product. By silica gel column chromatography, eluting with ethyl acetate:petroleum ether (1:3), 111.2 mg of the target compound was obtained as light yellow oil, with a yield of 85.5%. 1 HNMR (300MHz, CDCl 3 ), δ (ppm): 6.85 (s, 1H, H Ar ), 6.83(s, 2H, H Ar )6.32(t, 1H, J=7.8Hz, OCH-), 3.94~3.84(s, 12H, 4XOCH 3 ), 2.12(s, 3H, OAc) 1.93~1.71(m, 5H, OAc, CH 2 ), 1.41 (s, 3H, CH 3 ), 1.39 (s, 3H, -CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com