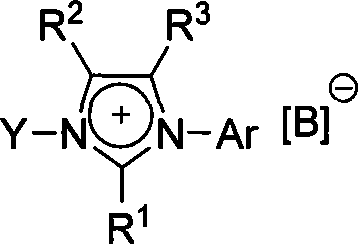
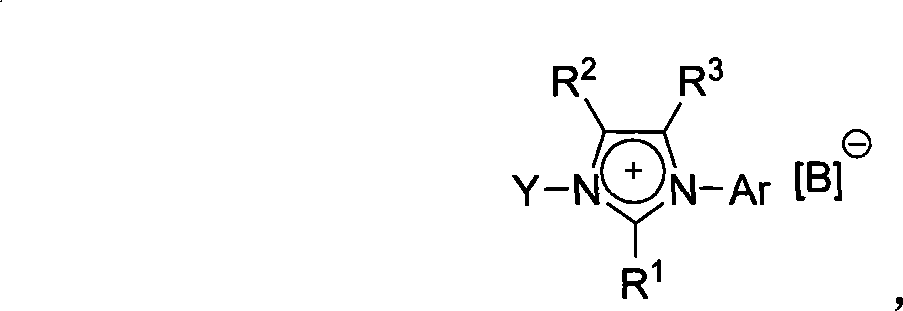
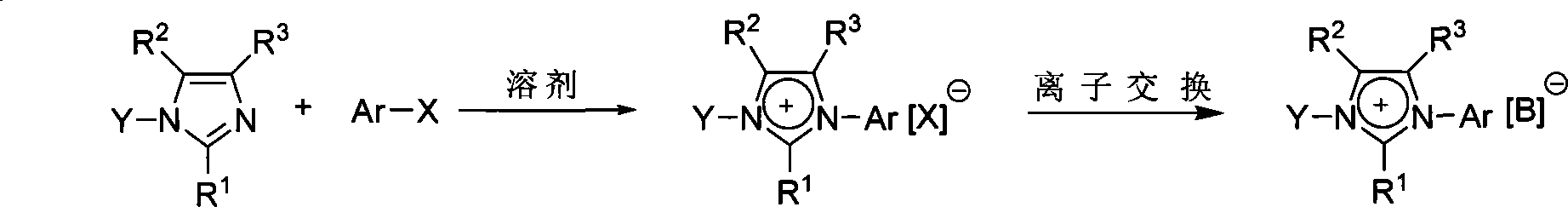
Imidazole-like ionic salt with electron donor-acceptor structure, preparation method and use thereof
A push-pull electron and ionic salt technology, applied in the field of nonlinear optical materials and ionic salts, can solve the problems of limited practical application, poor processability, low thermal stability, etc., and achieves simple reaction and post-processing operations, high product purity, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of N-(p-methoxyphenyl)-N'-(2,4-dinitrophenyl) imidazole chloride salt
[0028]
[0029]Add 1 mmol of N-(p-methylphenyl)imidazole, 1.1 mmol of 2,4-dinitrochlorobenzene and 1 ml of acetonitrile into the sealed tube, tighten the plug of the sealed tube, and stir the reaction in an oil bath at 110°C for 4 hours. After the heating was stopped and the reaction system was cooled, it was filtered, and the filter residue was washed several times with ether to obtain a white solid product. Yield 90%.
[0030] 1 HNMR (CD 3 SOCD 3 ) δ (ppm) 10.37 (s, 1H, --- NH), 9.06 (d, 1H, J = 2.4Hz), 8.91 (d-d, 1H, J = 2.4Hz, J = 8.7Hz), 8.55 (S, 1H) 8.40(s, 1H), 8.37(s, 1H), 7.83(d, 2H, J=8.4Hz), 7.23(d, 2H, J=8.4Hz), 3.85(s, 3H, OCH 3 ) MS (ESI) M / Z (%) 314.2 (M + )IR (KBr) v3402, 3140, 3021, 2886, 2771, 1849, 1665, 1618, 1508, 1358, 1250, 1078, 1017, 953, 897, 860, 773, 662, 530
[0031] UV (ethanol, alcohol): λ (max) = 265nm
[0032] EA. Found: C, 50.78;...
Embodiment 2
[0033] Embodiment 2: the preparation of N-phenyl-N'-(2,4-dinitrophenyl) imidazolium chloride salt
[0034]
[0035] Add 1 mmol of N-phenylimidazole, 1.1 mmol of 2,4-dinitrochlorobenzene and 1 ml of acetonitrile into the sealed tube, tighten the plug of the sealed tube, and stir the reaction in an oil bath at 110°C for 2 hours. After the heating was stopped and the reaction system was cooled, it was filtered, and the filter residue was washed several times with ether to obtain a white solid product. Yield 82%.
[0036] 1 HNMR (D 2 O) δ (ppm) 9.21 (s, 1H), 8.77 (d, 1H, J = 9.6HZ), 8.09 (br, 2H,) 7.95 (s, 1H), 7.61 (m, 5H) MS (ESI) M / Z(%)311.2(M + )IR (KBr) v3029, 2878, 2785, 1859, 1607, 1549, 1494, 1457, 1350, 1260, 1147, 1085, 899, 837, 778, 760, 738, 688, 653, 521.
[0037] UV (alcohol): λ (max) = 237nm
[0038] EA. Found: C, 52.00; H, 3.24; N, 16.38; Theoretical: C, 51.96; H, 3.20; N, 16.16.
Embodiment 3
[0039] Embodiment 3: Preparation of N-(p-methylphenyl)-N'-(2,4-dinitrophenyl) imidazolium chloride salt
[0040]
[0041] Add 1 mmol of N-(p-methylphenyl) imidazole, 1.1 mmol of 2,4-dinitrochlorobenzene and 1 ml of acetonitrile into the sealed tube, tighten the plug of the sealed tube, and stir the reaction in an oil bath at 110°C for 12 hours. After the heating was stopped and the reaction system was cooled, it was filtered, and the filter residue was washed several times with ether to obtain a white solid product. Yield 87%.
[0042] 1 HNMR (D 2 O) δ (ppm) 9.21 (s, 1H), 8.78 (d, 1H, J = 8.4Hz), 8.11 (d, 1H, J = 8.4Hz) 8.07 (S, 1H), 7.97 (s, 1H) 7.54 (d, 2H, J=8.4Hz), 7.56(d, 2H, J=8.4Hz) 2.36(s, 3H, CH 3 )
[0043] MS(ESI)M / Z(%)325.2(M + )
[0044] IR(KBr)v3495, 3247, 3167, 3058, 2778, 1983, 1774, 1621, 1508, 1357, 1242, 1067, 956, 911, 815, 739, 651, 624, 523
[0045] UV (alcohol): λ (max) = 238nm
[0046] EA. Found: C, 53.16; H, 3.68; N, 15.31; Theoretical: C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com