Tissue engineering ligament recovering material and its preparation method
A repair material and tissue engineering technology, applied in the field of medicine and biomedical engineering, can solve the problems of high price, complicated surgical operations, and the necessity of correction, and achieve the effect of good biomechanical performance and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The preparation method of the tissue engineered ligament repair material of the present invention is simple, a certain amount of ligament cells are mixed with a pharmaceutically acceptable biodegradable material, and then the surface of the guide body is combined with ligament healing promoting factors with optimized controlled release kinetics or / and ligament fibroblasts or stem cells.
[0049] The shape of the tissue-engineered ligament graft of the present invention is not particularly limited, and can be shaped arbitrarily according to the shape of tissue defect.
[0050] The diameter of the nano minimally invasive guide in the present invention is preferably less than 500 nm.
[0051] The concentration of ligament cells or bone marrow stromal stem cells in the tissue engineered ligament graft of the present invention is usually 0.5×10 6 / ml to 5×10 8 / ml or higher, preferably 5×10 6 / ml to 5×10 8 / ml.
[0052] In addition, in the tissue engineered tendon graft...
Embodiment 1
[0056] Example 1 The acquisition of bone marrow stromal stem cells
[0057] Seeding cells is an essential element in tissue engineering research. The application of tissue engineering technology to construct ligament tissue requires the expansion and culture of as few cells as possible in vitro to reach the number of cells required for the construction of tendon tissue.
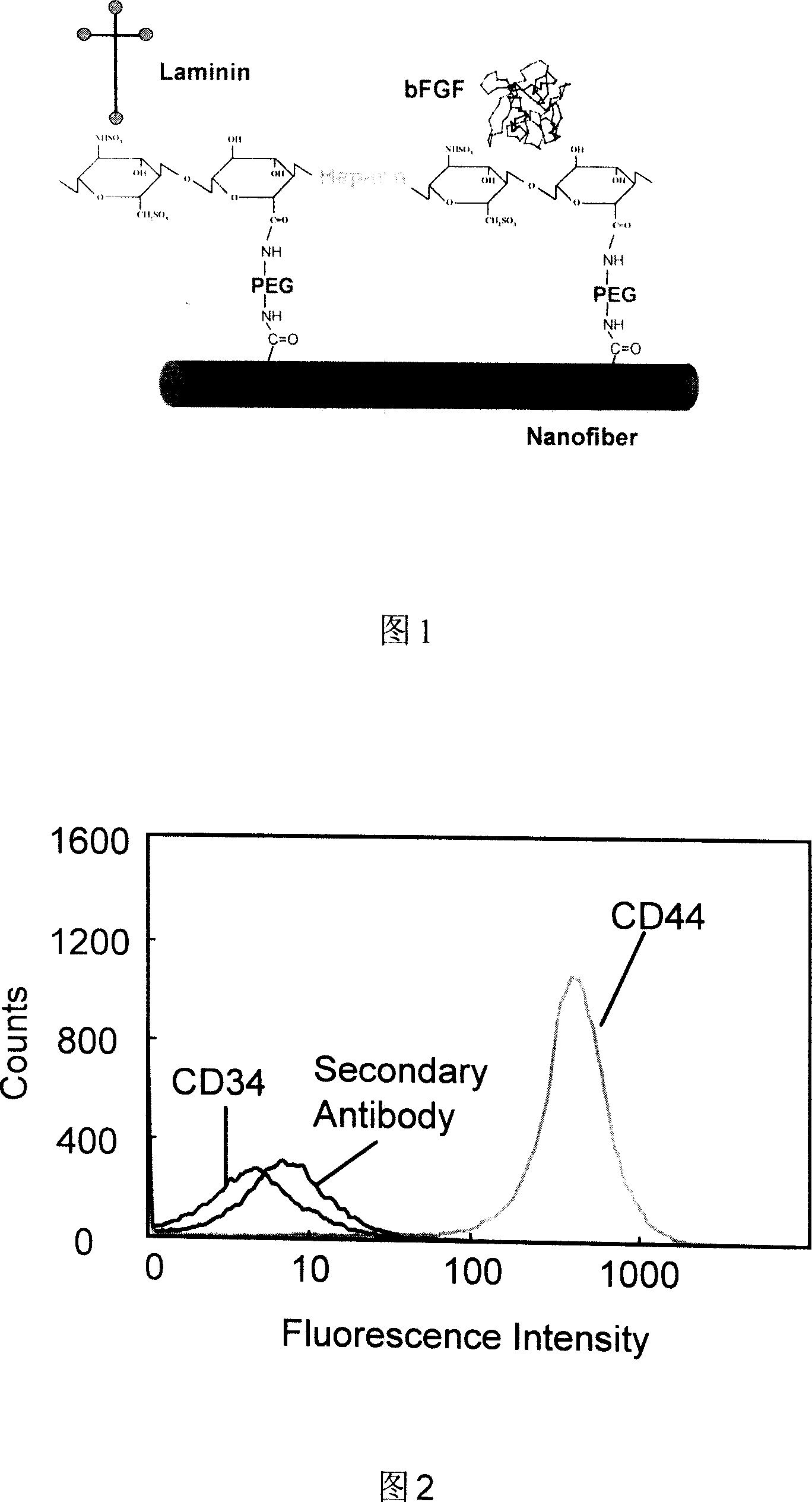
[0058] Bone marrow stromal stem cells were obtained from Cambrex (Walkersville MD), and the markers on the cell surface were positive for CD44 and negative for CD34 by flow cytometry (see Figure 2), so the purified bone marrow stromal stem cells were isolated. They were cultured in MSCGM medium (Cambrex), saturated humidity, 5% CO2, 37°C in a constant temperature shaker.
[0059] The subculture steps of bone marrow stromal stem cells include:
[0060] Aspirate all the culture medium in the culture dish, wash it with PBS; add 3ml of 0.25% trypsin, put it in a 37°C incubator for digestion for 2 minutes; obser...
Embodiment 2
[0062] Preparation of embodiment 2 import body
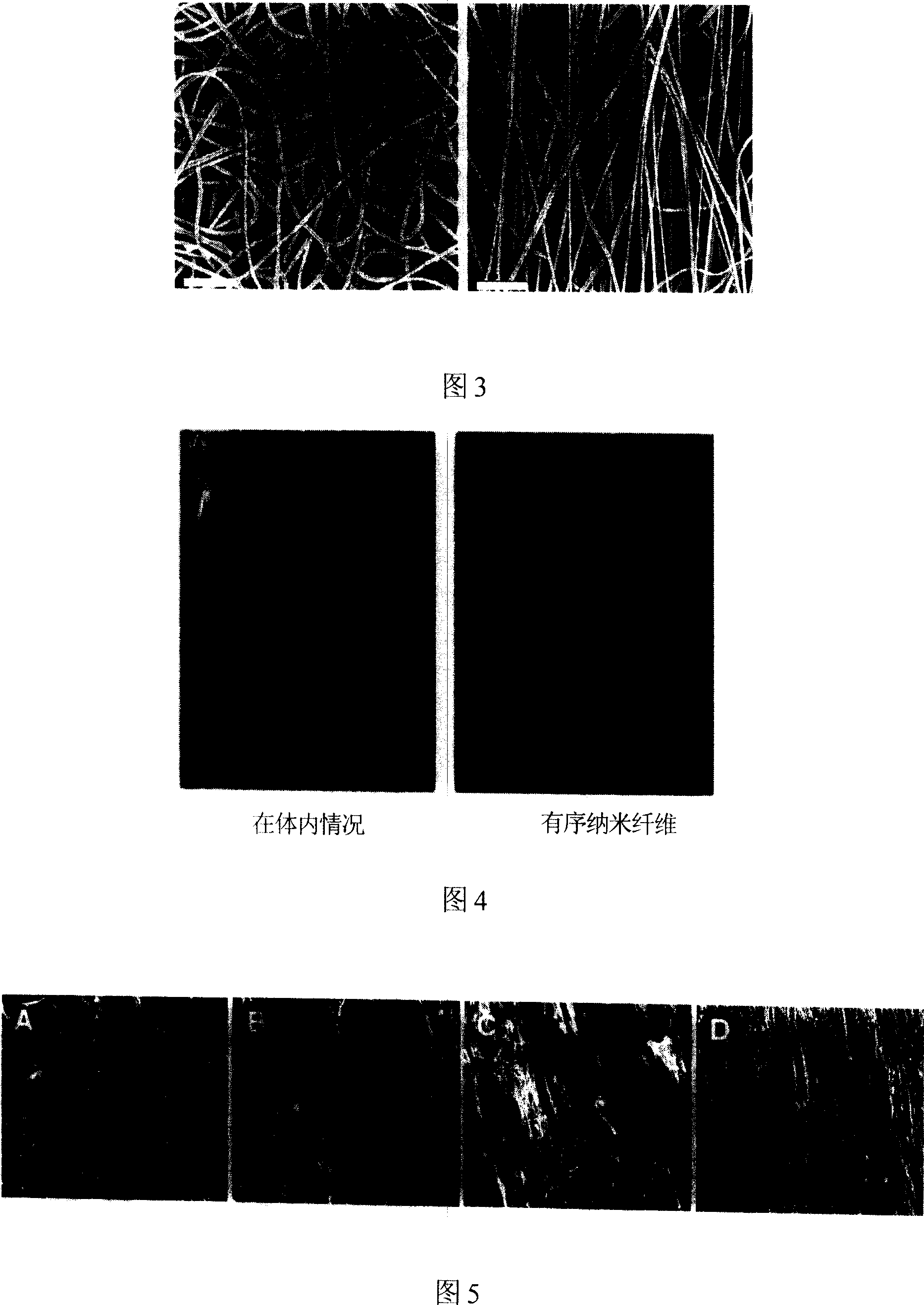
[0063] After the copolymer of PLA and PGA is sprayed by an electroplating spinning forming apparatus, and then subjected to a uniaxial stretching apparatus, the nanofiber filaments are arranged in an orderly manner to form an aggregate of materials for the import body.
[0064] Fig. 3 is a scanning electron microscope image of the fabricated nano-stent, in which the left image shows that the nano-fiber filaments are disorderly arranged to form a nano-introducer. The picture on the right shows that after uniaxial stretching, the nanofiber filaments are arranged in an orderly manner to form a nanointroducer. It is best to use nanofiber aggregates arranged orderly after uniaxial stretching for the introduction body.
[0065] The preparation of the above-mentioned import body is an important aspect of tissue engineering technology. In the past, carbon fibers and braids were used as scaffolds in tendon tissue engineering. However,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com