Peg-polyacetal and peg-polyacetal-poe graft copolymers and pharmaceutical compositions
A technology of graft copolymer and diol, which is applied in the field of controlled-release pharmaceutical compositions, can solve the problems of graft copolymer systems that do not disclose thermal gel graft copolymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0479] Preparation of graft copolymers
[0480] Graft copolymers can be prepared by methods known in the art, for example, as in Contemporary Polymer Chemistry, H.R. Allcock and F.W. Lampe, Prentice Hall, Inc. Englewood Cliffs, New Jersey 07632, 1981, and references cited therein described in the literature.
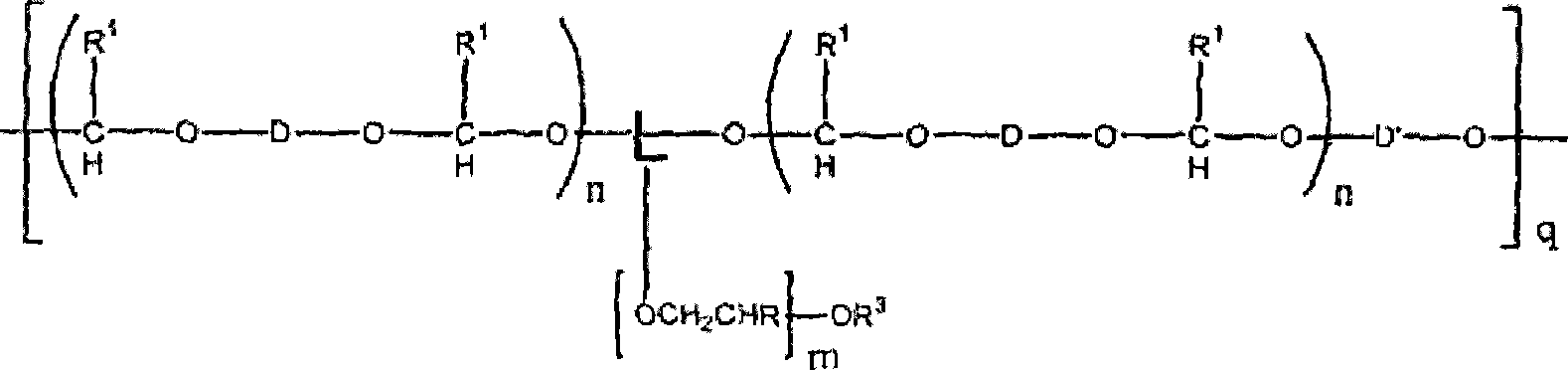
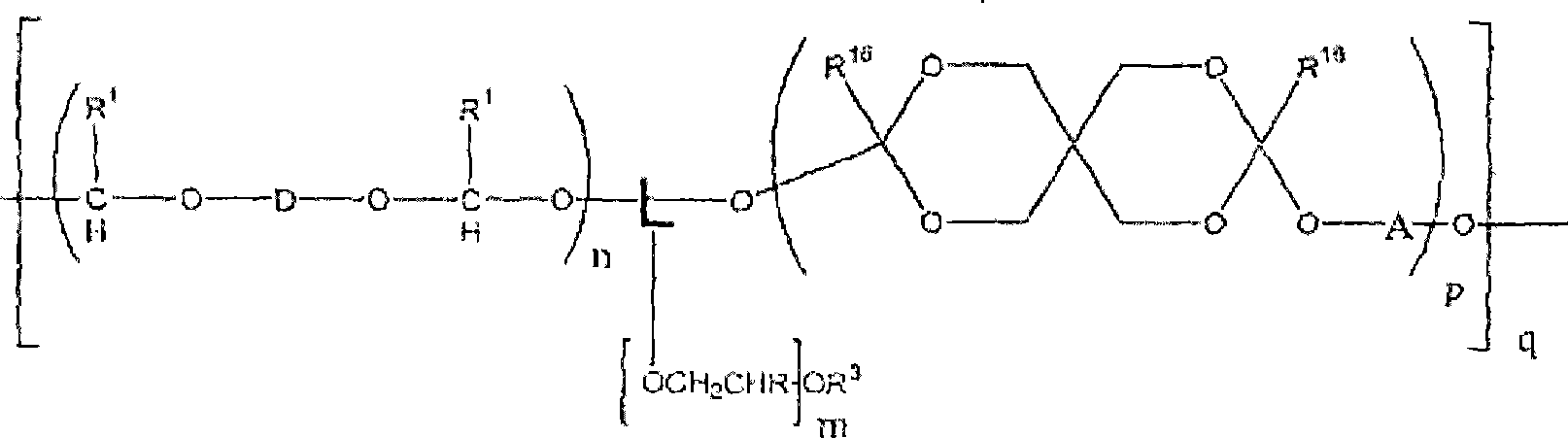
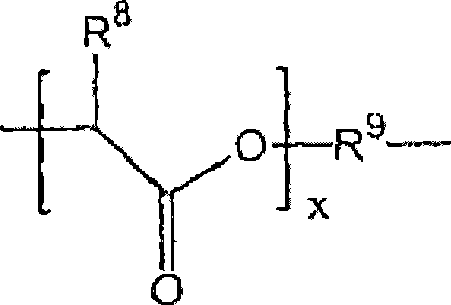
[0481] For example, polyacetal-polyethylene glycol graft copolymers of formula II can be prepared by reaction of divinyl ethers of formula IIa. In a particular aspect of the invention, a particular compound of divinyl ether of formula Ha is either commercially available or prepared by suitable methods known in the art. For example, depending on the identity of variable D, commercially available aminovinyl ethers can be combined with methyl esters to provide divinyl ethers of formula IIa. See US Patent Publication No. 2002 / 0082362 Al, Brocchini et al. Similarly, hydroxy vinyl ether compounds are commercially available and can be used with ester groups in the backbone t...
Embodiment 1
[0569] 9-Fmoc-protected 2-amino-1,3-propanediol (9-fluorenylmethoxycarbonyl-protected serinol) was synthesized by: 2 g (0.022 mol) 2-amino-1 , 3-propanediol (serinol) was dissolved in 54ml of 10% Na 2 CO 3 in solution. 10 ml of dioxane was added and the mixture was stirred in an ice bath. 7.38 g (0.0285 mol) of 9-fluorenylmethyl chloroformate (Fmoc-Cl) was dissolved in 25 ml of dioxane, and added dropwise to the above solution. The reaction mixture was stirred at room temperature for 4 hours. 200 ml of water were added and the product was extracted with ethyl acetate. The ethyl acetate layer was collected and washed with MgSO 4 dry. After filtration and solvent evaporation, the product was reprecipitated from ethyl acetate / hexanes and dried under vacuum.
[0570] PEG-N-succinimide carbonate (PEG-SC) was prepared by dissolving 1 mmol of α-methyl-ω-hydroxy polyethylene glycol (MPEG-OH) in 2 ml of acetonitrile and 0.4 ml of pyrimidine . 2 mmol of N,N'-disuccinimide carbo...
Embodiment 2
[0572] Polyacetal and grafted PEG synthesis was accomplished as follows:
[0573] The first step: the reaction is carried out in a dry box. 1 g (5.09 mmol) of 1,4-cyclohexane dimethylene divinyl ether, 0.5143 g (3.566 mmol) of 1,4-trans cyclohexane dimethylene and 0.4789 g (1.529 mmol) of 9 -Fmoc-protected serinol was dissolved in 6 ml of tetrahydrofuran. 0.34 ml of catalyst, p-toluenesulfonic acid (2% dissolved in tetrahydrofuran) was added by stirring, and the reaction was carried out for 4 hours.
[0574] Step 2: Take out the flask from the dry box and add a few drops of diisopropylethylamine to neutralize the acidic catalyst. The solution was diluted with 19 ml of tetrahydrofuran, and 5 ml of piperidine was added. A deprotection step was performed for 30 minutes, followed by dialysis for 24 hours in tetrahydrofuran (membrane with a molecular weight cut-off of 1000). The solvent was partially evaporated and the concentrated solution was precipitated in methanol. Polyac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com