Fusion protein penetrating blood-brain barrier with nerve nourishment gene and encoding gene and uses thereof
A neurotrophic factor and fusion protein technology, which is applied in the field of neurotrophic factor fusion protein, can solve the problems of bringing into the brain parenchyma, unstable conjugates, low yield of fusion protein, etc., and achieve the effect of exerting biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Design of BDNF-PTD / Antp fusion protein structure
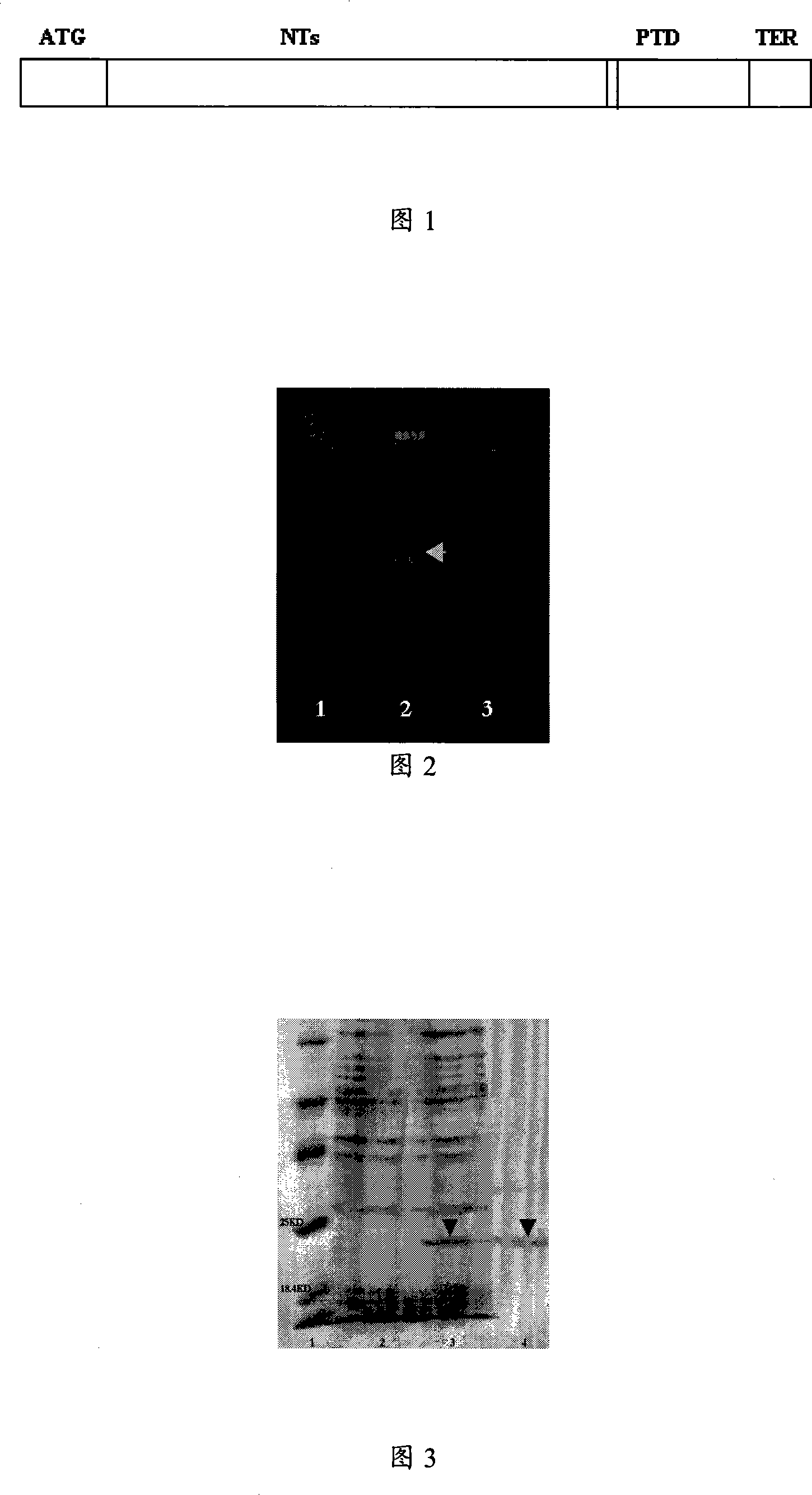
[0049]Please refer to Figure 1, ATG is the nucleotide encoding methionine, PTD is the protein transduction domain, NTs is the nucleotide sequence of the human neurotrophic factor family member encoding the mature domain protein, and TER is the termination codon of the nucleic acid. Methionine was added before the maturation region of BDNF, PTD / Antp was located at the C-terminal of BDNF, and it was connected with BDNF through the spacer amino acid glutamic acid to obtain the structure of BDNF-PTD / Antp fusion protein.
Embodiment 2
[0050] Example 2: Amplification of BDNF-PTD / Antp fusion protein gene and construction of prokaryotic expression vector
[0051] According to the nucleotide sequence of the coding BDNF mature region protein reported by the gene bank, and the structure of the above-mentioned BDNF-PTD / Antp fusion protein, the primers for amplifying the BDNF-PTD / Antp fusion protein gene are designed as follows:
[0052] Primer I is shown in SEQ ID NO: 3:
[0053] 5'GC CATATG CACTCTGACCCTGCCCGCCGA-3' (NdeI site is underlined)
[0054] Primer II is shown in SEQ ID NO: 4:
[0055] 5'-GC CTCGAG CTAGCATTTTTCCACTTCATCCTCCTGTTTTGGAATTGGAACCAGATTT
[0056] GTCTGCCGGATCCCCTTTTAATGGTCAATGTACATAC-3' (XhoI site is underlined)
[0057] Using the PCR method, the BDNF-PTD / Antp gene was amplified using the plasmid popeMPBDNF (ATCC, USA) containing the full-length cDNA of human BDNF as a template. The PCR reaction parameters were: pre-denaturation at 94°C for 3 min, denaturation at 94°C for 45 sec, annealin...
Embodiment 3
[0058] Example 3: Induced expression of BDNF-PTD / Antp fusion protein
[0059] Transform the competent expression strain BL21(DE3)plys with the correct pBDNF-PTD / Antp recombinant plasmid analyzed by enzyme digestion and nucleic acid sequencing, and spread it on a plate containing 50 mg / mL kanamycin and 34 mg / mL chloramphenicol. Pick a single positive clone at random, inoculate into 200mL LB medium test tubes containing kanamycin (50mg / mL) and chloramphenicol (34mg / mL), cultivate overnight at 37°C, and transfer to 1000mL containing the same Continue to culture in LB medium with antibiotics for 1 hour, to OD 600 When = 0.6, add IPTG to a final concentration of 0.1 mmol / L, induce culture for 5 hours, centrifuge at 5000 rpm for 15 min at 4°C, and discard the supernatant to collect the bacteria.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com