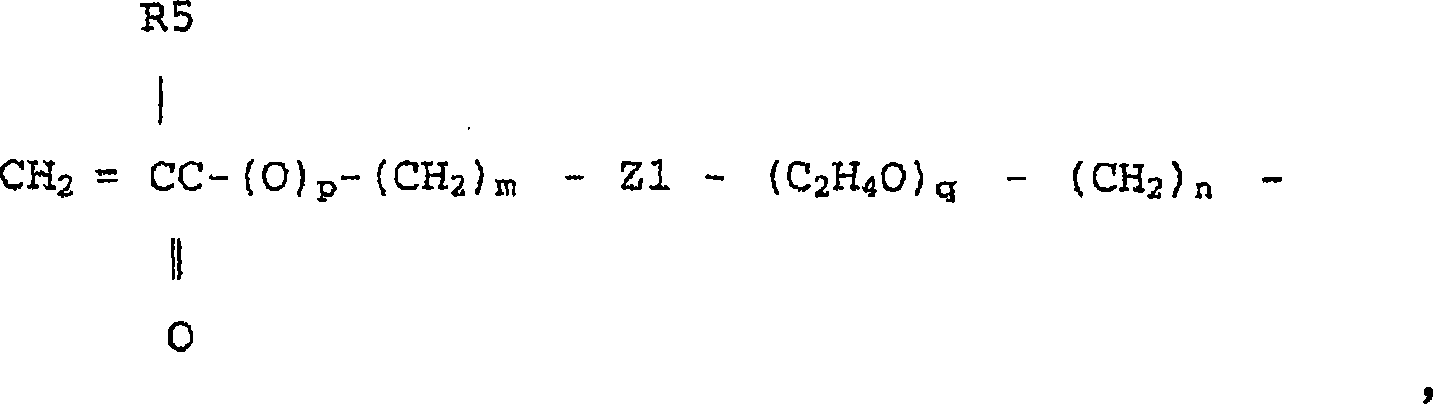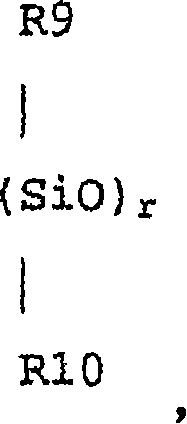Silicone hydrogel contact lens
A contact lens and silicon hydrogel technology, applied in the direction of instruments, optics, optical components, etc., can solve the problems that polarographic technology cannot provide measured values, glasses are not very wettable, and glasses are stiff
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
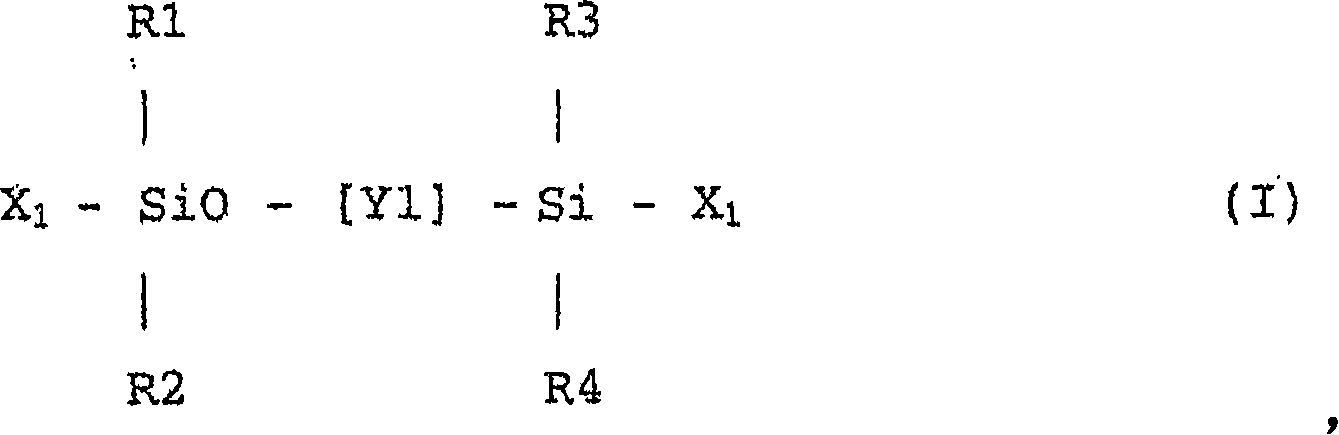
[0108] An example of a useful synthetic method includes the step of reacting cyclic siloxanes having hydrosilyl groups (Si—H), cyclic siloxanes having hydrocarbyl Mixtures of siloxanes and disiloxanes having hydroxyalkyl groups at both ends and (in some cases) cyclic siloxanes having fluorine-substituted hydrocarbyl groups are subjected to ring-opening polymerization to obtain A hydrosilyl group-containing polysiloxane compound having a hydroxyl group. In this case, silicon with various degrees of polymerization and introduction ratios of fluorine-containing substituents to hydrosilyl groups can be obtained by changing the feeding ratio of each cyclic siloxane to disiloxane compound used. Oxygen compounds.
[0109] Isocyanato-substituted acrylates or isocyanato-substituted methacrylates are then reacted with hydroxyl groups at the end of the polysiloxane to obtain urethane-containing fluorinated urethanes with polymerizable unsaturated groups at both ends. Silicone compounds...
example
[0156] The following non-limiting examples illustrate various aspects and features of the invention.
Synthetic example 1
[0158] [Synthesis of polysiloxane diol (A1) having a hydrosilyl group]
[0159]At 25°C, 150 grams of octamethylcyclotetrasiloxane, 22.6 grams of 1,3,5-trimethyltrifluoropropyl-cyclotrisiloxane, 5.2 grams of 1,3,5,7-tetra Methyl-cyclotetrasiloxane, 9.8 grams of 1,3-bis(3-(2-hydroxyethoxy)propyl)tetramethyldisiloxane, 200 grams of chloroform and 1.5 grams of trifluoromethanesulfonic acid The mixture was stirred for 24 hours, followed by repeated washing with purified water until the pH of the mixture became neutral. After separating water, chloroform was distilled off under reduced pressure. The residual liquid was dissolved in acetone (36 g), reprecipitated with methanol (180 g), and the volatile components were removed from the separated liquid under vacuum to afford a clear viscous liquid. The liquid was siloxanediol (H3R) having a hydrosilyl group represented by the following formula, and the yield thereof was 125 g. Here, although the structural formula of the linker Y i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| modulus | aaaaa | aaaaa |
| modulus | aaaaa | aaaaa |
| modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com