Nonaqueous electrolyte solution, electrochemical energy storage device using same, and nonaqueous electrolyte secondary battery
A non-aqueous electrolyte, dialkoxyethane technology, applied in the field of non-aqueous electrolyte secondary batteries, can solve the problems of discoloration, insolubility of lithium salts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
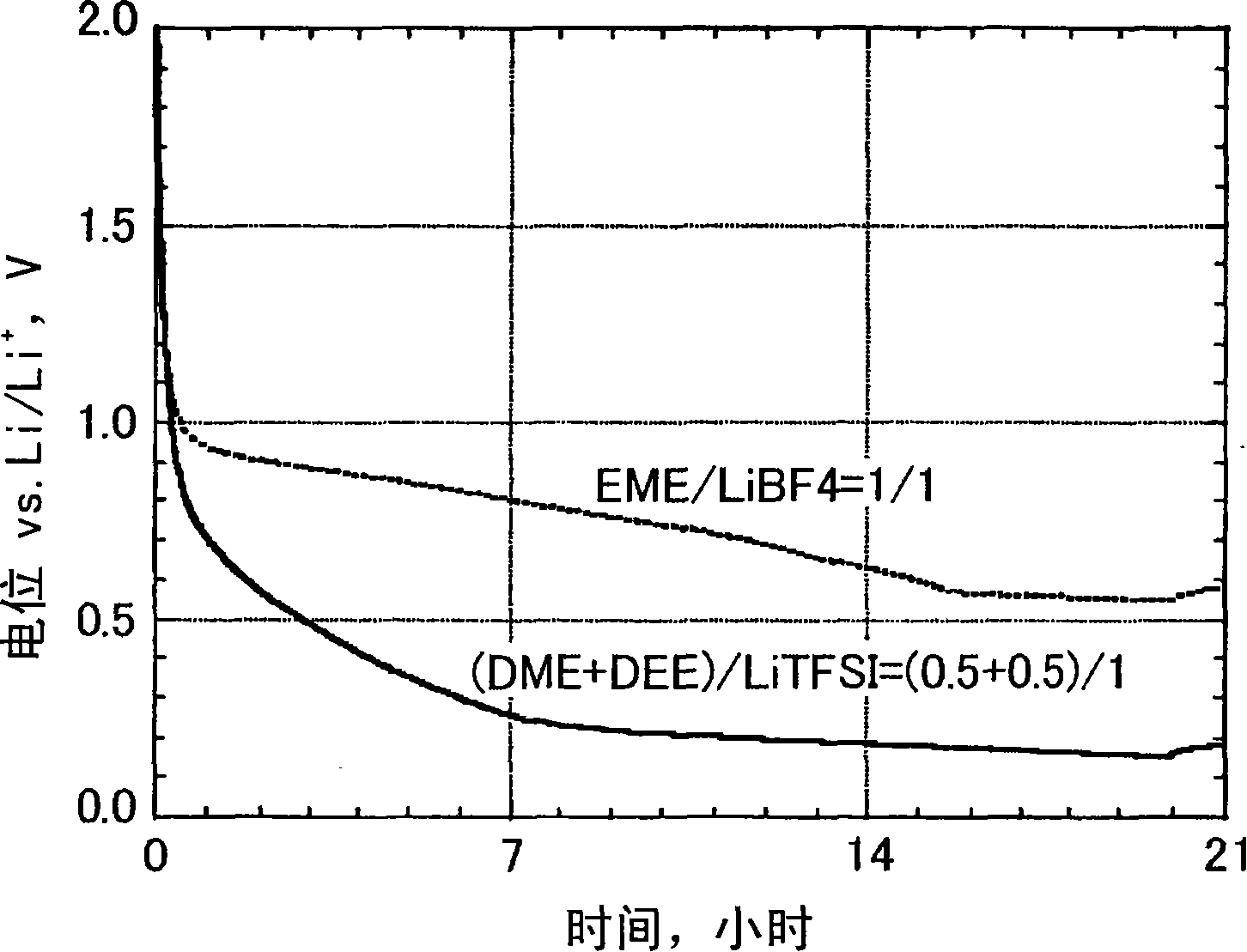
[0047] The effects of different non-aqueous electrolyte solutions on high-temperature stability according to the type of lithium salt were studied. DME, DEE and LiTFSI are mixed in a ratio of (0.5+0.5) / 1 according to the molar ratio of (DME+DEE) / LiTFSI, so as to prepare a non-aqueous electrolyte. The obtained liquid was transparent at normal temperature.
[0048] The prepared non-aqueous electrolyte solution is filled into a container made of tetrafluoroethylene-perfluoroalkyl vinyl ether copolymer resin (hereinafter referred to as PFA) and tightly closed, and then the PFA container is stored in an aluminum laminated bag, and then sealed . After the container was stored at 60° C. for 10 days, changes in the color tone of the nonaqueous electrolyte solution were investigated. As a result, the nonaqueous electrolytic solution of Example 1 maintained a transparent state.
Embodiment 2
[0058] LiTFSI and various 1,2-dialkoxyethanes were mixed in ratios of various molar ratios. Table 2 shows the composition of the non-aqueous electrolytic solution which is liquid at normal temperature. Various nonaqueous electrolytic solutions prepared were stored at 60° C. for 10 days in the same manner as in Example 1, and changes in color tone after storage were investigated.
[0059] Table 2
[0060] color change
DEE / LiTFSI=0.75 / 1
none
(DME+DEE) / LiTFSI=(0.5+0.5) / 1
none
(DME+ETFEE) / LiTFSI=(0.5+0.5) / 1
none
(DME+BTFEE) / LiTFSI=(0.6+0.4) / 1
none
(EME+DEE) / LiTFSI=(0.2+0.8) / 1
none
DEE / LiTFSI=1 / 1
none
(DEE+MTFEE) / LiTFSI=(0.8+0.2) / 1
none
(DEE+ETFEE) / LiTFSI=(0.5+0.5) / 1
none
(DEE+BTFEE) / LiTFSI=(0.7+0.3) / 1
none
DME / LiTFSI=2 / 1
none
DEE / LiTFSI=2 / 1
none
DPE / LiTFSI=2 / 1
none ...
Embodiment 3
[0066] In the non-aqueous electrolytic solution of the present invention, whether lithium ions can be intercalated into the graphite material was investigated as follows.
[0067] Artificial graphite powder (MAG-D manufactured by Hitachi Chemical Co., Ltd.) can be used as the negative electrode active material for intercalating / deintercalating lithium ions by charging and discharging.
[0068] The negative electrode plate was produced by the following method. First, 75 parts by mass of artificial graphite powder, 20 parts by mass of acetylene black as a conductive agent, 5 parts by mass of polyvinylidene fluoride resin as a binder, and dehydrated N-methyl-2-pyrrolidone as a dispersion solvent to mix. Next, the mixture was coated on one surface of a 20 μm thick copper foil current collector and dried to form an 80 μm thick active material layer. Then, the copper foil current collector formed with the active material layer was cut into a size of 35 mm × 35 mm, and a copper cur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com