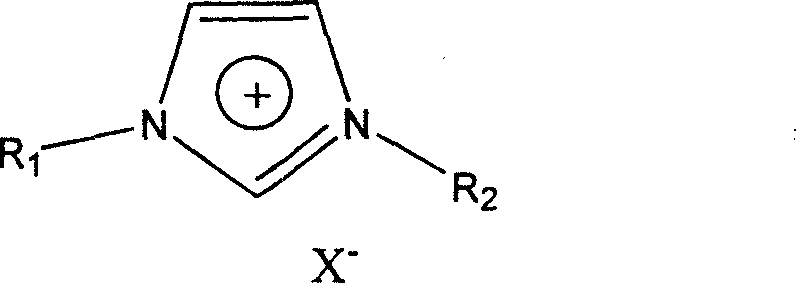
Preparation method for fluorochlorobenzene
A chlorobenzene and fluorine substitution technology is applied in the field of preparation of aromatic fluorine-containing organic compounds, which can solve problems such as catalyst failure to catalyze, waste water pollution, etc., and achieve the effects of high conversion rate and yield, simple product separation, and obvious economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of preparation of fluoropentachlorobenzene, put 500ml there-necked bottle and stir, add 0.04mol hexachlorobenzene, 0.28mol anhydrous potassium fluoride, 150ml ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate, The reactants were mixed and stirred.
[0047] React at 160°C to 230°C for 16 hours. During the reaction, the temperature increases gradually. Starting from 160°C, the temperature gradually rises until it reaches 230°C at the end of the reaction. Then, it is separated by vacuum distillation to obtain 9.12 grams of fluoropentachloro Benzene C 6 Cl 5 F, productive rate is 85% (in hexachlorobenzene); Obtain 1.1 grams of difluorotetrachlorobenzene C simultaneously 6 Cl 4 f 2 , and the yield was 11% (calculated as hexachlorobenzene).
Embodiment 2
[0049] A preparation of fluoropentachlorobenzene, put a 500ml three-necked bottle on it and stir, add 0.04mol hexachlorobenzene, 0.28mol anhydrous potassium fluoride, 180ml ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate , and the reactants were mixed and stirred.
[0050] React for 12 hours at 160°C to 230°C. During the reaction, the temperature rises gradually, and the heating rate can be roughly uniform, but it is not necessary to strictly follow the constant rate of temperature rise; vacuum rectification and separation obtain 8.63 grams of fluoropentachlorobenzene C 6 Cl 5 F, productive rate is 80% (in hexachlorobenzene); Obtain a small amount of difluorotetrachlorobenzene C simultaneously 6 Cl 4 f 2 (<1%).
Embodiment 3
[0052] A kind of preparation of fluoropentachlorobenzene, put 500ml there-necked bottle and stir, add 0.04mol hexachlorobenzene, 0.28mol anhydrous potassium fluoride, 200ml ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate, The reactants were mixed and stirred.
[0053] Reacted at 210°C for 10 hours, and separated by vacuum distillation to obtain 9.04 grams of fluoropentachlorobenzene C 6 Cl 5 F, productive rate is 84% (in hexachlorobenzene); Obtain 0.8 gram of difluorotetrachlorobenzene C simultaneously 6 Cl 4 f 2 , and the yield was 8% (calculated as hexachlorobenzene).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com