Use of calcitonin as combined treatment therapy for the management of inflammatory disease conditions
A technology for inflammatory diseases and calcitonin, applied in allergic diseases, drug combinations, skin diseases, etc., can solve problems such as weakened anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
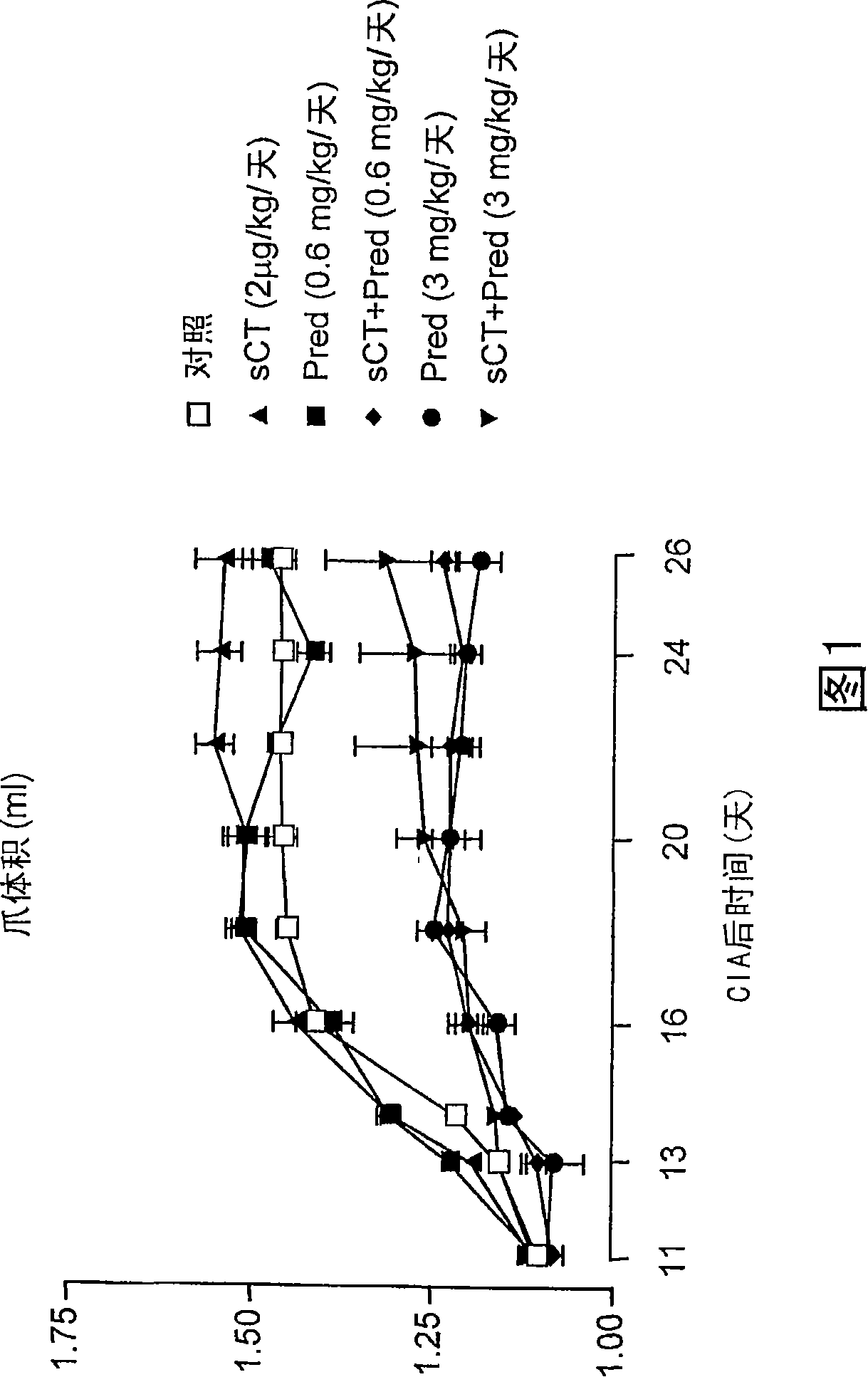
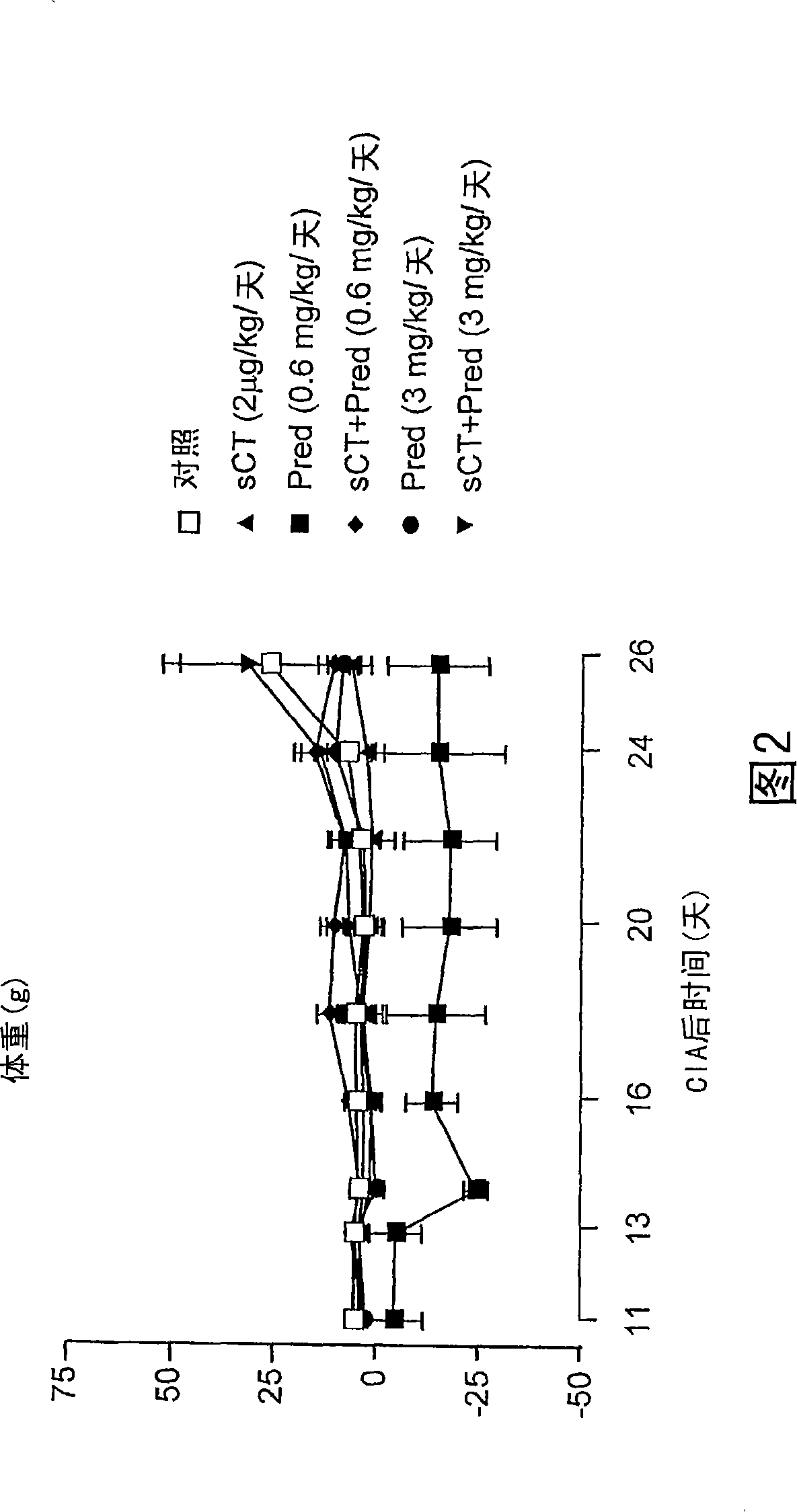
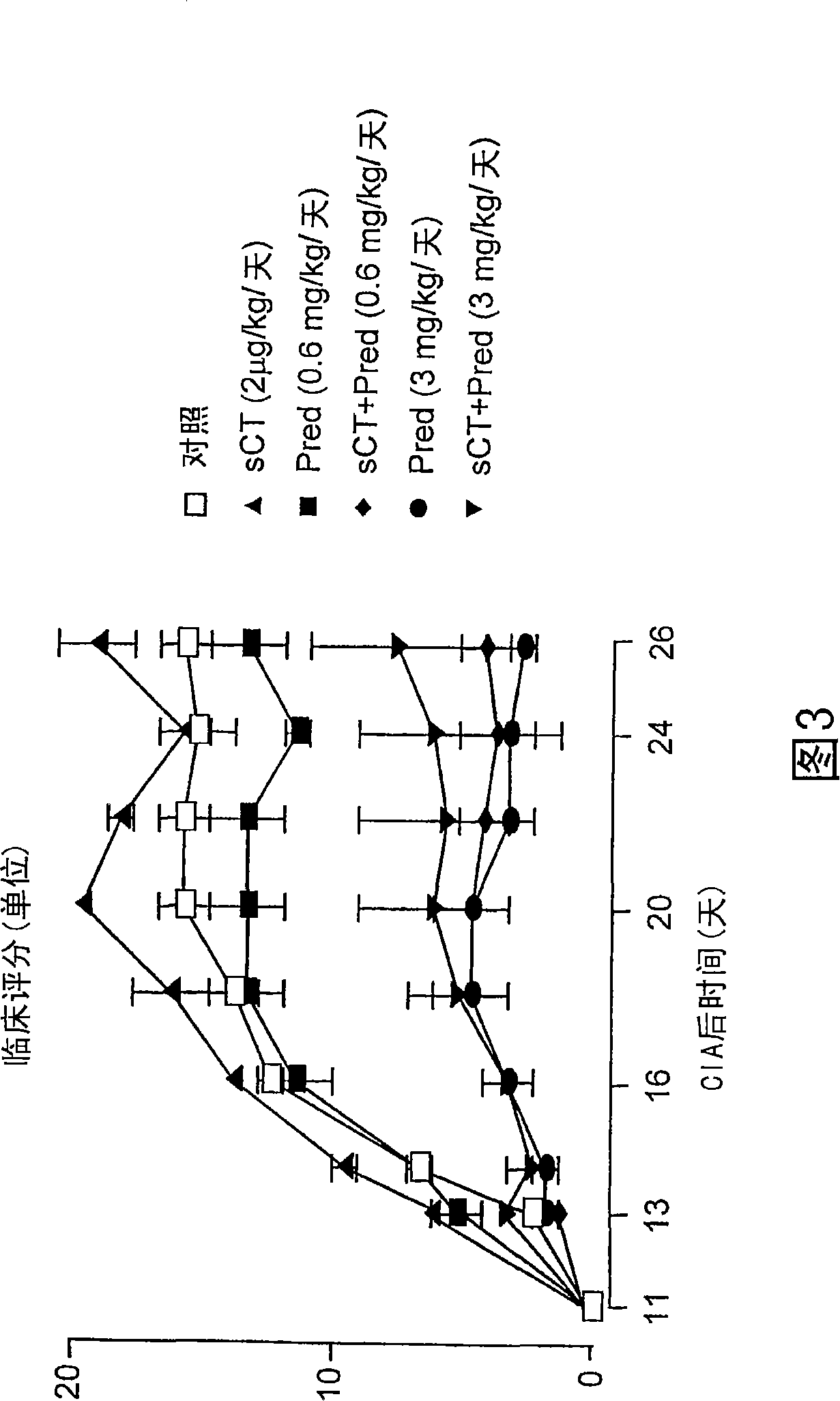
[0087] Example 1: A Study of the Effect of a Treatment Regime for Experimentally Induced Arthritis
[0088] Collagen II-induced arthritis (CIA). Female Lewis rats (body weight 150±20g; HarlanUK Ltd Bicester, Oxfordshire, England) were fed with standard feed, free to drink water, and kept in a 12-hour light / dark cycle. Animal manipulations were performed under license from the Home Office under the Animals Act (Scientific Procedures, 1986). Bovine nasal collagen II (4 mg / ml; Sigma-Aldrich Ltd, Poole, UK) was dissolved in acetic acid (0.01 M) and emulsified with an equal volume of ice-cold incomplete Freund's adjuvant (Sigma-Aldrich). On day 0, rats were anesthetized with halothane, the tail tip was clipped, and collagen II / adjuvant suspension (400 μg collagen II per mouse) was intradermally injected. On days 11 to 13, the first symptoms of arthritis were evident, and the strongest inflammatory response was observed on days 18 to 21.
[0089] CIA-induced inflammation is limit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com