Pharmaceutical composition for curing osteoarthritis
A kind of osteoarthritis and composition technology, applied in the field of compositions for treating osteoarthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
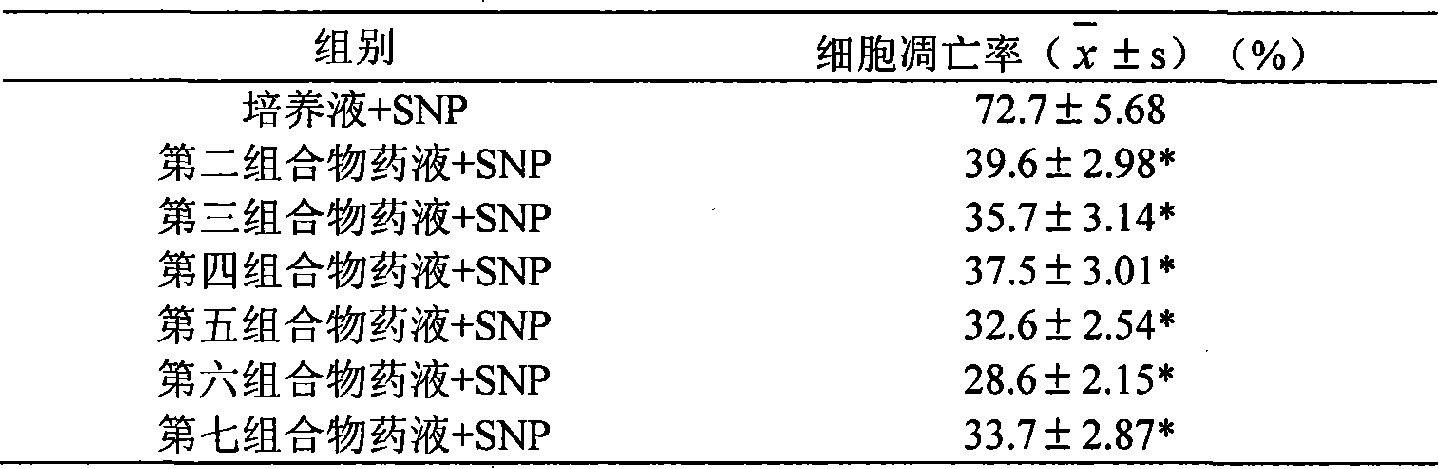
[0027] Embodiment 1 The influence of the present invention on the apoptosis of chondrocytes caused by nitroprusside
[0028] Reagents and drugs: type II collagenase, trypsin, dimethyl sulfoxide, blue tetrazolium (MTT), toluidine stain, DMEM medium, fetal bovine serum, nitroprusside (SNP)
[0029] Animals: 2-3 days old clean grade New Zealand rabbits for cell culture
[0030] Preparation, culture and identification of rabbit chondrocytes in vitro: take newborn New Zealand rabbits, kill them and disinfect them with alcohol, cut off the limbs of young rabbits under aseptic conditions on an ultra-clean workbench, and separate the muscles and joint capsules around the shoulders, elbows and knee joints. Cut unossified cartilage tissue, put it into a petri dish, add a little 10% FBS DMEM culture solution, cut it into 1mm×1mm with ophthalmic scissors, and centrifuge (1500r / min) for 5min at room temperature. After removing the supernatant, move the cartilage tissue into a flask, add d...
Embodiment 2
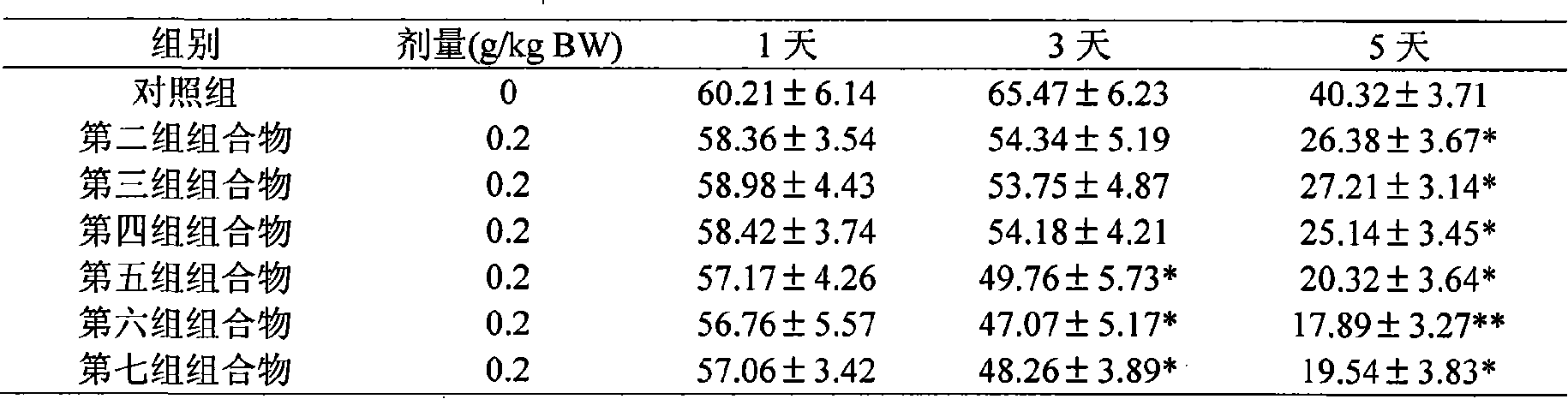
[0037] Embodiment 2 Composition of the present invention induces arthritis experiment on rat kaolin
[0038] Animals: 70 female rats with a body weight of 130-150 g were randomly divided into 7 groups, which were respectively the control group and the second to seventh raw drug compounding experimental groups. The control group was administered with normal saline, and the experimental group was given a dose of 0.2g / kg BW
[0039] Experimental method: intragastric administration of the test substance, 1 hour later, conventional disinfection of the ankle toe joint of the right hind limb and injection of 0.2ml of 10% sterilized kaolin normal saline suspension, 1, 3, 5 days after injection, by volumetric measurement method The volume (ml) of the left and right hind paws was measured. Give the test substance every day, and after 5 days, inject 1% Evans blue into the tail vein according to the amount of 1ml / kg BW, and 2 hours after the injection, kill the animal after ether anesthe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com