Method for inhibiting influenza virus infection and medicament thereof
A technology for influenza virus infection and drugs, applied in the direction of viral peptides, antiviral agents, viruses/phages, etc., can solve the problems of interfering with the virus adsorption process, drug-resistant strains, and hindering the binding of HA to host cell membrane surface receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
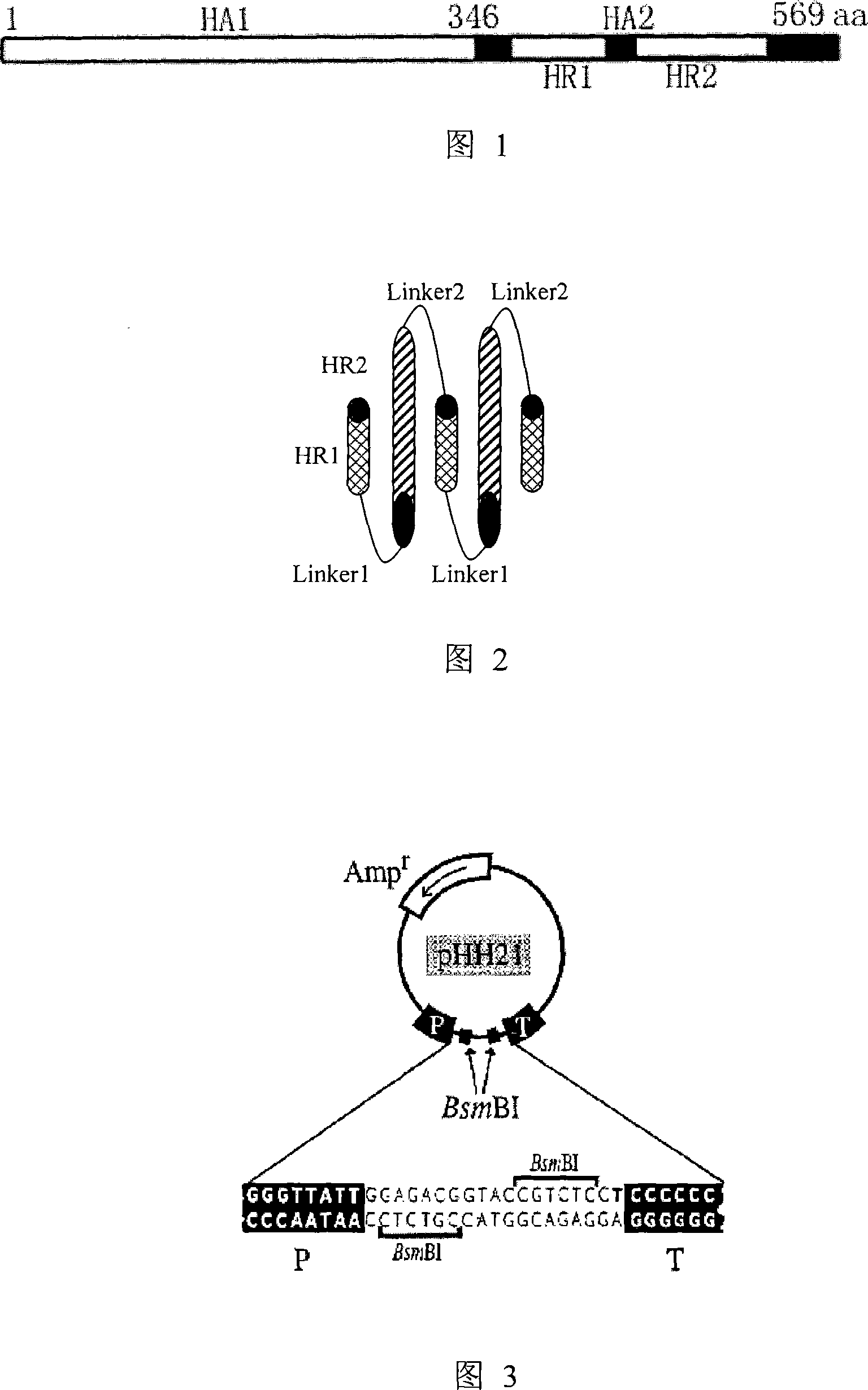
[0055] Example 1: Construction of viral RNA expression plasmid of HA and NA genes of highly pathogenic avian influenza virus A / bar-headed goose / Qinghai / 1 / 05 strain
[0056] 1.1) Extraction of viral RNA
[0057] The highly pathogenic avian influenza virus A / bar-headed goose / Qinghai / 1 / 05 strain (hereinafter referred to as the QH strain) is a highly pathogenic avian influenza virus of the H5N1 subtype, which was isolated and preserved by our laboratory (refer to Liu et al. al, 2005). The QH strain influenza virus chicken embryo allantoic fluid stored at -70°C was centrifuged at 3000r / m for 10min to remove impurities, and then 140μl of supernatant was pipetted into a 1.5ml centrifuge tube without RNase, and viral RNA extraction kit (QIAmp Viral RNA Mini Kit, CAT. No. 52904) extract viral RNA and operate according to the instructions provided by the kit. After extraction, adsorption, washing, centrifugation, and elution, 60μl of viral RNA solution was obtained, which was stored at -80°...
Embodiment 2
[0073] Example 2: Preparation of recombinant influenza virus WSN and QH-WSN
[0074] 2.1) Preparation of recombinant influenza virus WSN
[0075] The recombinant human influenza virus A / WSN / 33 (H1N1 subtype, sometimes referred to as "WSN") used in the experiment was prepared by our laboratory using a 12-plasmid influenza virus reverse genetic system (Luytjeset al, 1989). The 12 This plasmid reverse genetic system was a gift from Professor Yoshihiro Kawaoka from the University of Wisconsin (Neumann et al, 1999). The system includes expression plasmids for expressing 8 negative-strand RNA fragments of influenza virus genome (including pHH21-PB2, pHH21-PB1, pHH21-PA, pHH21-HA, pHH21-NP, pHH21-NA, pHH21-M, pHH21 -NS,) and four protein expression plasmids expressing influenza virus replicase complex (including pcDNA3-PB2, pcDNA3-PB1, pcDNA3-PA, pCAGGS-NP). The above plasmids were transformed into competent cells of Escherichia coli DH5α, and the single clones were picked and cultured in ...
Embodiment 3
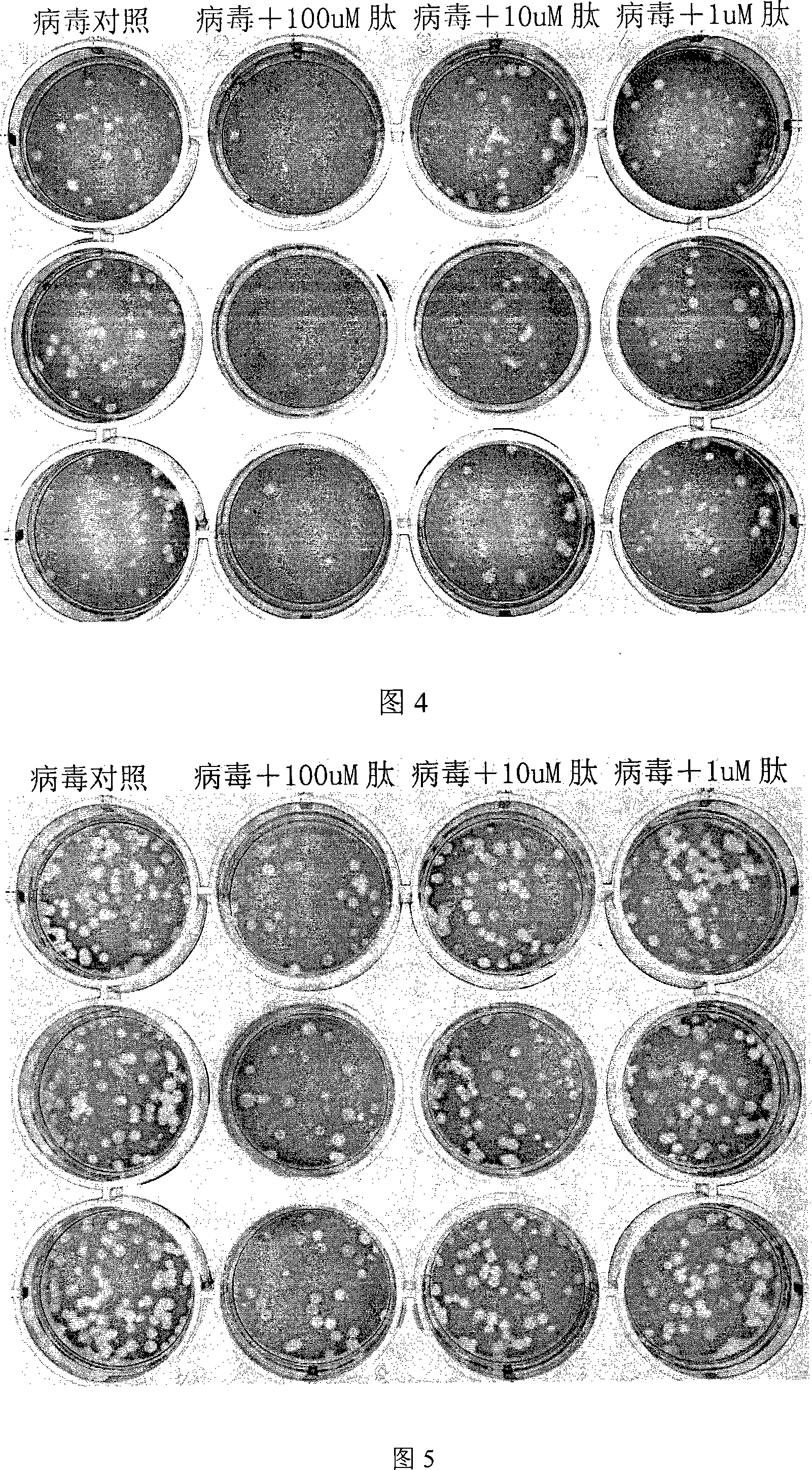
[0078] Example 3: Determination of plaque titer of experimental influenza virus
[0079] In order to test the inhibitory effect of the polypeptide and protein of the present invention on the highly pathogenic avian influenza virus, we tested its inhibitory effect on the QH-WSN recombinant virus with the H5N1 highly pathogenic avian influenza virus infection characteristics. At the same time, in order to test whether the above-mentioned peptides and proteins have cross-inhibition effects on human influenza viruses, we tested their inhibitory effects on recombinant WSN viruses (human influenza viruses) with similar genetic background as QH-WSN but with a surface membrane protein of H1N1 subtype. ; In addition, we also tested its cross-inhibitory effect (blue rain) against the representative strain of human influenza virus H3N2 subtype isolated from southern China in 2005 (A / Jiangxi / 312 / 2005, referred to as "JX" strain) Et al., 2006).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com