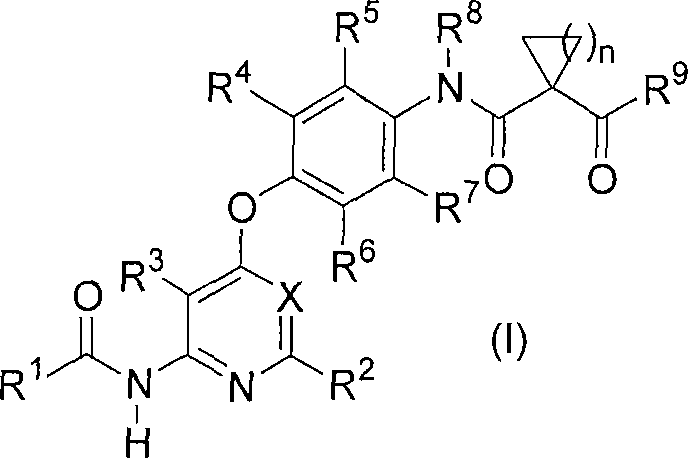
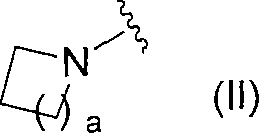
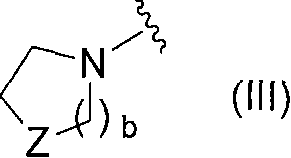Novel pyridine derivative and pyrimidine derivative (3)
A technology of compounds and hydrates, applied in drug combinations, cardiovascular system diseases, organic chemistry, etc., can solve problems such as unreported and unrecorded compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0466] This step is a step of obtaining compound (1t) from compound (1ps) (compound (1ps) is compound (1p) and compound (1s) described in [Production method 1-B].). The same method as can be used.
[0467]
[0468] This step is a step of obtaining compound (1u) from compound (1t). The same method as can be used.
[0469]
[0470] This step is the compound (1u) 2 protecting groups " R 102 -O-C(=O)-" and "P" are deprotected to obtain the compound (1x). According to the type of protecting group, the following reactions can be appropriately combined to carry out this step, thereby obtaining the compound (1x), namely Deprotection reactions using acids such as hydrochloric acid and trifluoroacetic acid; deprotection reactions using inorganic bases such as sodium hydroxide and potassium hydroxide; deprotection reactions using tetrabutylammonium fluoride, etc.; by using palladium carbon, palladium hydroxide Such as the deprotection reaction carried out by the catalytic hydrog...
Embodiment
[0647] The compounds of the present invention can be produced, for example, according to the methods described in the following Preparations and Examples. However, these are illustrative examples, and the compounds of the present invention are not limited to the following specific examples in any way.
[0648] Unless otherwise specified, YMCSIL-60-400 / 230W was used as silica gel for purification in the preparation examples and examples.
[0649] In addition, unless otherwise specified, any one of the following two conditions (gradient elution condition 1 or gradient elution condition 2) was used as the LC-MS purification conditions.
[0650] ODS column (CAPCELL PAK C-18)
[0651] Solvent Liquid A Water
[0652] Solvent Liquid B Acetonitrile
[0653] Solvent C liquid 1% trifluoroacetic acid aqueous solution
[0654] Flow rate 30ml / min
[0655] end time 10min
[0656] Gradient elution condition 1
[0657] 0.00min A: 80%, B: 10%, C: 10%
[0658] 7.80min A: 30%, B: 60%, C:...
preparation example 1
[0665] (Preparation Example 1) tert-butyl 3-dimethylaminoazetidine-1-carboxylate
[0666]To a solution of 1-Boc-azetidin-3-one (3.45 g) in methanol (175 ml) was added 2M dimethylamine-tetrahydrofuran solution (21.9 ml), acetic acid (1.73 ml), 10% palladium on carbon ( 2.15 g), stirred at room temperature in a hydrogen atmosphere for 14 hours. The catalyst was filtered off, and the filtrate was concentrated under reduced pressure. The residue was partitioned between ethyl acetate-saturated aqueous sodium bicarbonate. The combined organic layers were dried over anhydrous sodium sulfate. It was concentrated to obtain the title compound (4.07 g, 101%) as a colorless oil.
[0667] 1 H-NMR spectrum (CDCl 3 )δ (ppm): 1.43 (9H, m), 2.17 (6H, s), 3.01 (1H, m), 3.79 (2H, m), 3.91 (2H, m).
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com