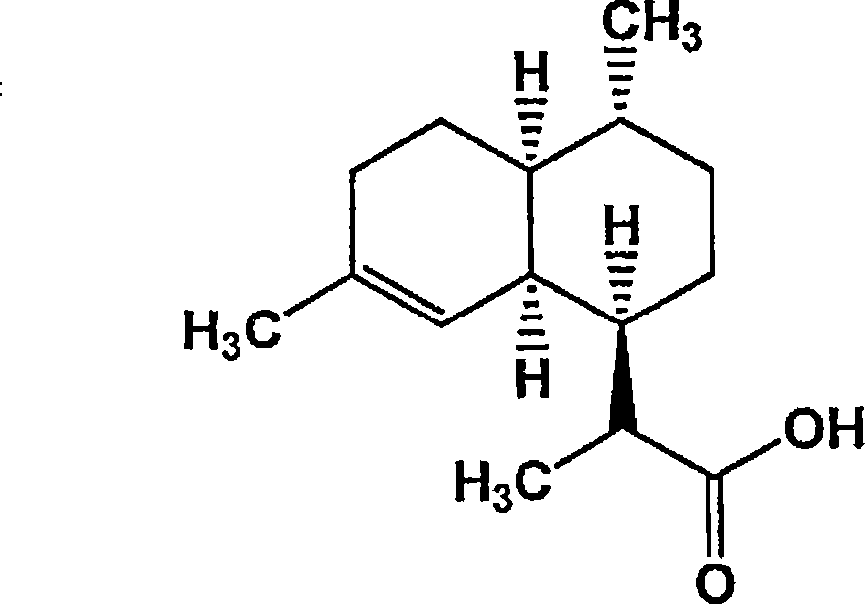
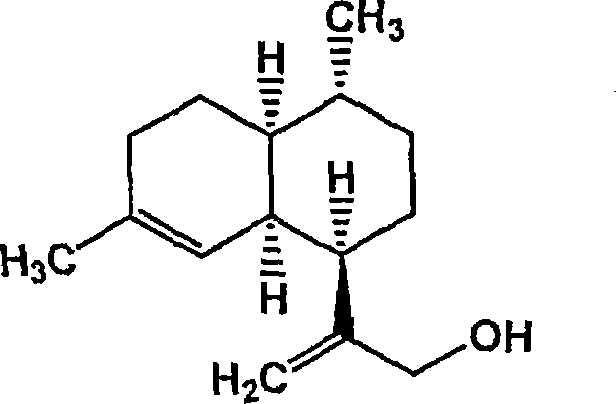
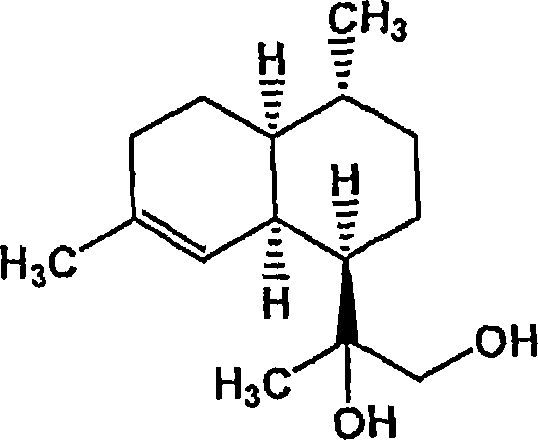
Conversion of amorpha-4,11- diene to artemisinin and artemisinin precursors
A kind of technology of amorpha and dihydroartemisinic acid, applied in the direction of organic chemistry and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
1.1.1 to 5 conversion
[0135] To a 250 mL flask equipped with a septum inlet and a magnetic stir bar, add 50 mmol of BH 3 SM 2 and 18 mL of freshly distilled THF. It was cooled to 0° C. and 115 mmol of cyclohexene were added dropwise. After stirring the mixture for 1 hour at 0 °C, (C 6 h 11 ) 2 BH was isolated as a white solid.
[0136] To (C 6 h 11 ) 2 To BH (solid, 50 mmol), 75 mmol of amorphadiene 1 was added. The reaction mixture was stirred for one hour at -25°C, then it was left in the refrigerator for one day. The trialkylborane was treated with 50 mL of 3N sodium hydroxide, 7.5 mL of 30% hydrogen peroxide and the reaction mixture was stirred at 25°C for 5 hours. The product was then extracted with ether and dried over sodium sulfate. Ether was then evaporated. The residue was filtered through silica gel (petroleum ether:ethyl acetate 9:1 as eluent) to remove olefins and cyclohexanol, then eluted with petroleum ether:ethyl acetate (1:1) to give Pure alcoho...
Embodiment 2
Transformation from 2.1.5 to 3
[0137] By adding sulfuric acid (17 mL) dropwise to CrO in water 3 The Jones reagent was prepared by cooling the solution (200 mmol), and the resulting solution was diluted with water until the total volume of the solution was 60 mL.
[0138] Alcohol 5 (65 mmol) was dissolved in acetone (150 mL) and cooled to 0 °C. Jones reagent was added dropwise through the dropping funnel over a period of 2 hours until the orange-brown color of the reagent persisted. The reaction mixture was stirred for another 2 hours. Diethyl ether was then added to precipitate chromeous salts. The reaction mixture was filtered and the residue was washed with ether. The organic layer was dried over anhydrous sodium sulfate, concentrated and purified by adding 5% aqueous sodium hydroxide. The product was washed with ether to remove impurities. The aqueous layer was acidified and extracted with ethyl acetate. The extract was dried over anhydrous sodium sulfate and conce...
Embodiment 3
3.1. Transformation of 1 into 3 via 9, 6 and 5
[0139] In a 250 mL three-necked flask equipped with a thermometer, condenser and magnetic stirring bar, 50 mmol of calcium hypochlorite and 50 mL of water were added, and while adding amorphadiene 1 dissolved in 200 mL of dichloromethane over 30 minutes, Stir vigorously. Stirring was continued for 3 h while 50 g of dry ice was added in small portions at regular intervals. The cloudy white slurry was filtered to remove inorganic salts. These inorganic salts were washed with two 25 mL portions of dichloromethane. The filtrate and washings were combined, the aqueous layer was decanted and the organic layer was dried over anhydrous sodium sulfate. The organic layer was filtered to remove the drying agent and concentrated under vacuum to give (9, X=Cl). The chlorine on 9 is hydrolyzed by boiling the concentrate with a 50:50 mixture of dioxane and water to give the unsaturated alcohol 6 after concentration. The unsaturated alcohol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com