Method for preparing medicine targeted controlled-release nano eyedrop
A technology of nanoparticles and eye drops, which can be used in drug combinations, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of low therapeutic concentration, adverse reactions, frequent, etc. The effect of prolonging the duration of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
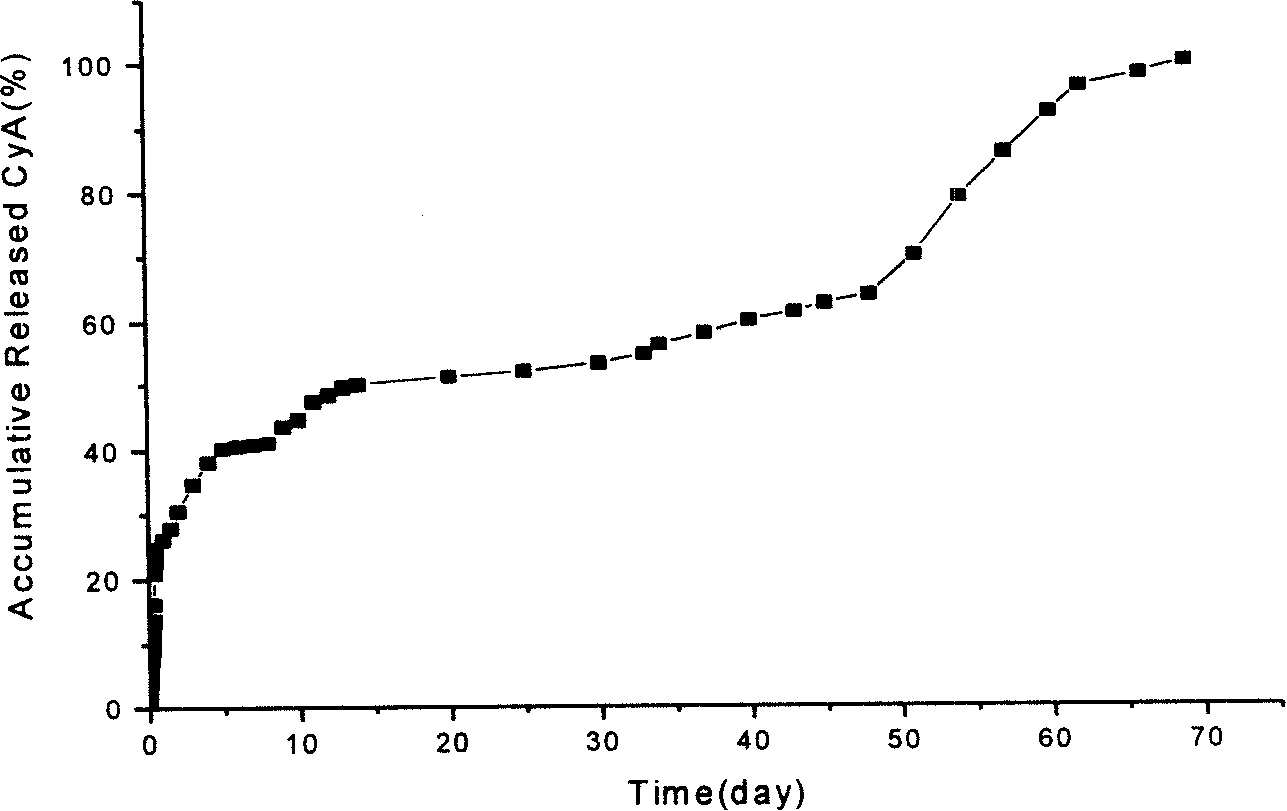
[0022] The present invention will be further described below in conjunction with the accompanying drawings and embodiments.
[0023] 1. Preparation of Eye Drops
[0024] (1) Weigh 2 grams of polylactic acid and 2 grams of cyclosporine A with an electronic balance, dissolve the two in 20ml of acetone, stir and mix well as the oil phase for later use.
[0025] (2) Take 3 grams of cholesterol-modified chitosan and 12 ml of deionized water with an electronic balance, dissolve the cholesterol-modified chitosan in deionized water under magnetic stirring, continue stirring for 10 hours, and the resulting solution is used as the water phase for subsequent use. The stirring speed is 1000 rpm.
[0026] (3) Inject the water phase into the oil phase under magnetic stirring at a rotational speed of 1200 rpm, so that the system becomes a uniformly dispersed O / W emulsion.
[0027] (4) Continue stirring until the acetone is completely volatilized to obtain an emulsion containing solidified ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com