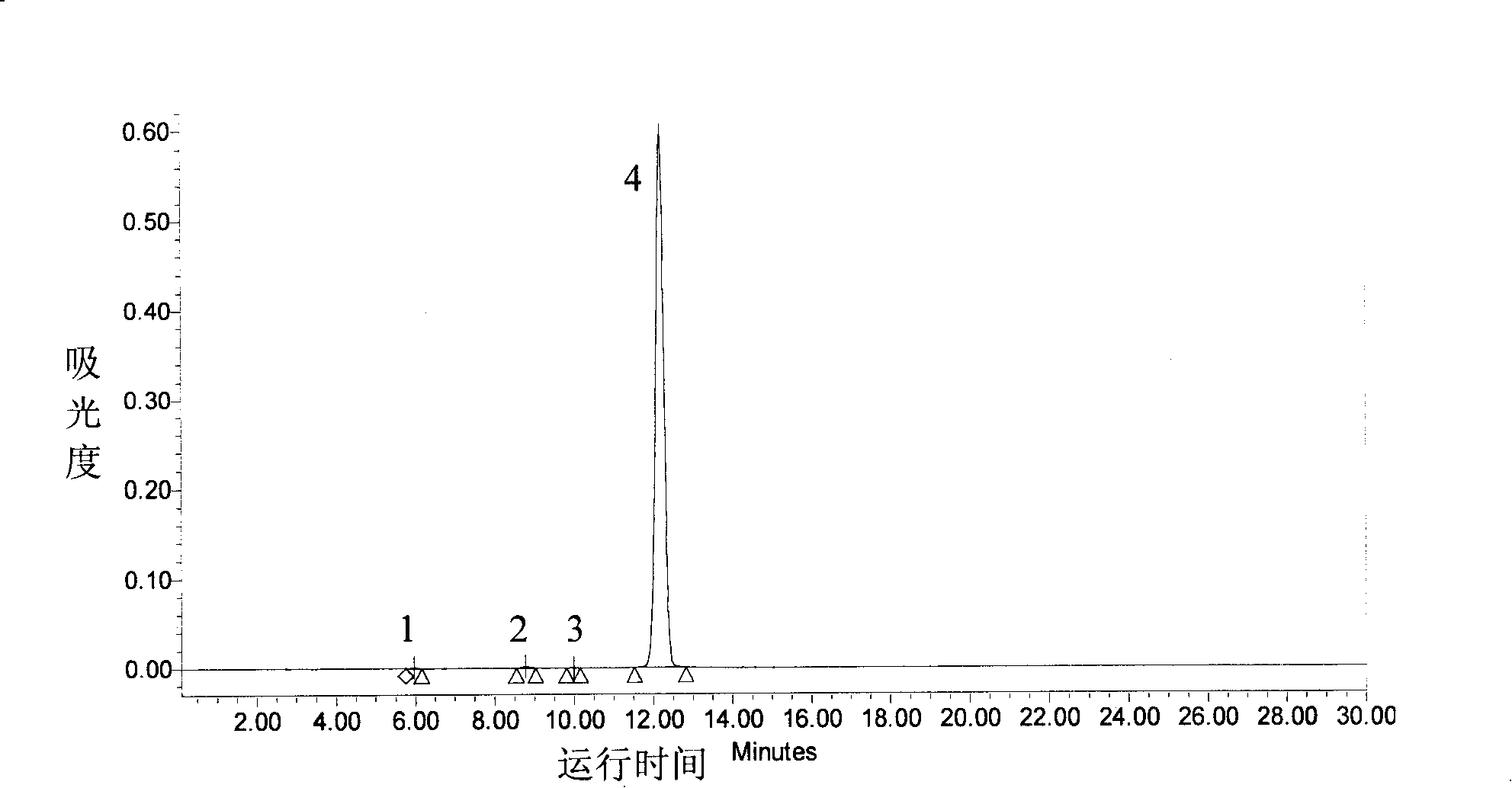
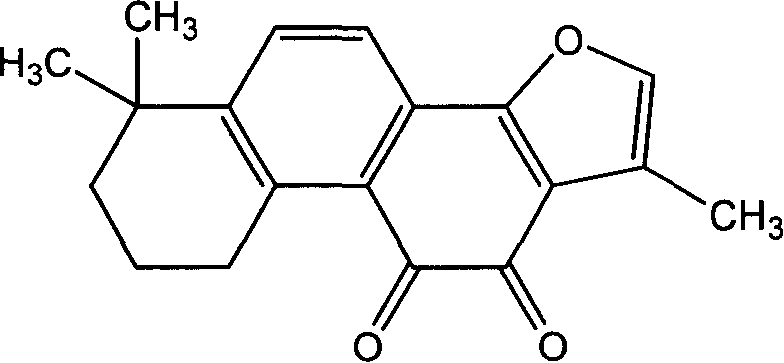
Method for separating and preparing tanshinone IIA chemical reference substance
A technology of tanshinone and reference substances, which is applied in the field of preparation of tanshinone IIA chemical reference substances, can solve the problems such as difficulty in stabilizing the purity of tanshinone IIA, difficulty in obtaining large quantities of tanshinone IIA chemical reference substances, lack of online monitoring means, etc., to shorten the separation time and conditions Gentle, easy-to-use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In this embodiment, 50% tanshinone IIA extract is used as raw material, 5 μm C18 bonded phase filler is used as adsorbent, and a solution containing methanol-water (75:25, volume ratio) is used as eluting solvent. The specific process steps are as follows:
[0025] 1. Sample pretreatment
[0026] The tanshinone IIA extract was dissolved in chloroform, configured into a tanshinone IIA solution with a concentration of 60 mg / ml, and filtered through a 0.22 μm microporous membrane.
[0027] 2. Column handling
[0028] The preparative HPLC column was selected as a C18 preparative column (5 μm, 20×250 mm) produced by Sheshido Company in Japan, and the chromatographic column was equilibrated with an elution system solution of about 5 times the column volume.
[0029] 3. Sample loading
[0030] The filtered sample solution was injected through the six-way valve, and the injection volume was 1ml.
[0031] 4. Elution
[0032] The flow rate was controlled at 10ml / min, and the ...
Embodiment 2
[0038] In this example, 70% salvianic acid B extract is used as raw material, 5 μm C18 bonded phase filler is used as adsorbent, and methanol-water (60:40, volume ratio) solution is used as eluting solvent. The specific process Proceed as follows:
[0039] 1. Sample pretreatment
[0040] The tanshinone IIA extract was dissolved in methanol, configured into a tanshinone IIA solution with a concentration of 8 mg / ml, and filtered through a 0.45 μm microporous membrane.
[0041] 2. Column handling
[0042] The preparative HPLC column is a self-packed preparative column. The filler is a C18 bonded phase filler with a particle size of 5 μm. The column is packed in a wet method. Equilibrate the column with an off-system solution.
[0043] 3. Sample loading
[0044] The filtered sample solution was injected through the six-way valve, and the injection volume was 8ml.
[0045] 4. Elution
[0046] The flow rate is controlled at 90ml / min, monitored at 270nm online, and the eluent c...
Embodiment 3
[0050] In this example, 60% tanshinone IIA extract is used as raw material, 10 μm of C18 bonded phase filler is used as adsorbent, and a solution containing methanol-water (75:25, volume ratio) is used as eluting solvent. The specific process steps as follows:
[0051] 1. Sample pretreatment
[0052] The tanshinone IIA extract was dissolved in ethanol, configured into a tanshinone IIA solution with a concentration of 10 mg / ml, and filtered through a 0.45 μm microporous membrane.
[0053] 2. Column handling
[0054] The preparative HPLC column is a self-packed preparative column. The filler is a C18 bonded phase filler with a particle size of 10 μm. The chromatographic column size is 75×250mm. The chromatographic column is equilibrated with an elution system solution about 10 times the column volume.
[0055] 3. Sample loading
[0056] The filtered sample solution was injected through the six-way valve, and the injection volume was 15ml.
[0057] 4. Elution
[0058] The fl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com