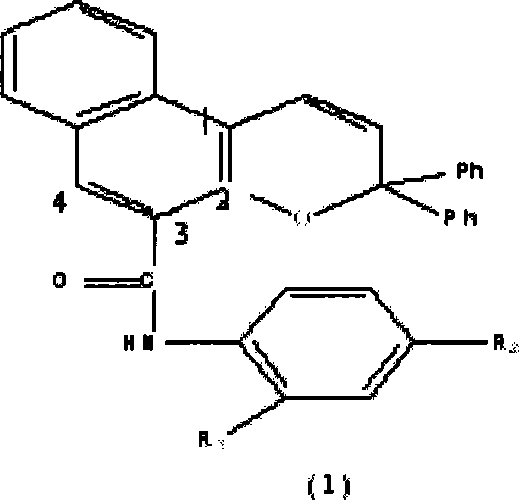
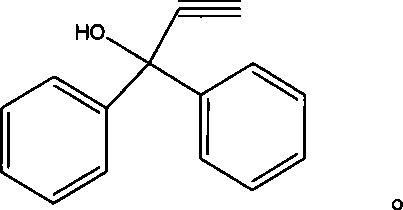
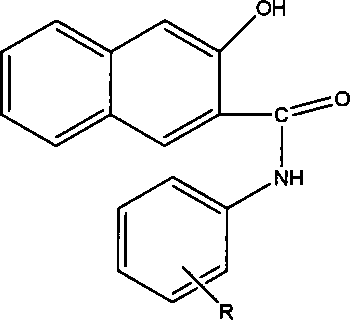
Photochromic naphtho-pyrans compounds and synthetic method thereof
A naphthopyran and photochromic technology, applied in chemical instruments and methods, color-changing fluorescent materials, etc., can solve problems such as complex synthesis routes, and achieve the effects of low price, excellent optical density and discoloration effect, and good fading rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 3.16g (0.012mol) SF1 in a 100ml round bottom flask, then add 50ml of toluene as a solvent, and then add 2.08g (0.01mol) 1,1-diphenyl propargyl alcohol and 0.17g (1mmol) For p-toluenesulfonic acid, stir magnetically, heat to 110°C and monitor the reaction by thin-layer chromatography. After 3 hours, a red reaction mixture is obtained, and the reaction is basically completed. Pour the reaction mixture into 100ml 10% NaOH aqueous solution, separate the organic layer, then wash once with 100ml 10% NaOH aqueous solution, then wash with water, spin dry the solvent on a rotary evaporator to obtain a red solid, and finally use petroleum ether: Ethyl acetate=12:1 eluent was passed through a silica gel column to obtain a light yellow solid pure product. Yield 43.5%.
[0033] MS (m / z): 453.2; IR (KBr, cm -1 ): 3337 (weak, aryl C-H bending out of plane), 3052, 3030 (medium strong, =C-H stretching), 1660 (strong, -C=C- stretching), 1650 (medium strong, stretching vibration),...
Embodiment 2
[0035] In 100ml round bottom flask, add 3.32g (0.012mol) SF2, then add 50ml dichloromethane as solvent, and then add 2.08g (0.01mol) 1,1-diphenyl propargyl alcohol and 0.17g ( 1 mmol) of p-toluenesulfonic acid, stirred by magnetic force, heated to 40° C. and monitored by thin-layer chromatography. After 2 hours, a red reaction liquid substance was obtained, and the reaction was basically completed. Pour the reaction mixture into 100ml 10% NaOH aqueous solution, separate the organic layer, wash once with 100ml 10% NaOH aqueous solution, then wash with water, spin dry the solvent on a rotary evaporator to obtain a light red solid, and finally wash with petroleum ether : Ethyl acetate = 20: 1 eluent passed through a silica gel column to obtain a light yellow solid pure product. Yield 45.2%. MS (m / z): 467.3; IR (KBr, cm -1 ): 3420 (weak, aryl C-H bent out of plane), 2913 (weak, -CH 3 stretching vibration), 1666 (strong, -C=C- stretching), 1650 (medium strong, stretching vibra...
Embodiment 3
[0037] Add 3.56g (0.012mol) SF3 in a 100ml round bottom flask, then add 50ml tetrahydrofuran as a solvent, and then add 2.08g (0.01mol) 1,1-diphenyl propargyl alcohol and 0.17g (1mmol) For p-toluenesulfonic acid, stir magnetically, heat to 66°C and monitor the reaction by thin-layer chromatography. After 4 hours, a gray reaction liquid substance is obtained, and the reaction is basically completed. Pour the reaction mixture into 100ml 10% NaOH aqueous solution, separate the organic layer, then wash once with 100ml 10% NaOH aqueous solution, then wash with water, spin dry the solvent on a rotary evaporator to obtain a gray solid, and finally use sherwood oil: Ethyl acetate=8:1 eluent was passed through a silica gel column to obtain a white solid pure product. Yield 50.6%. MS (m / z): 487.3; IR (KBr, cm -1 ): 3337 (weak, aryl C-H bends out of plane), 1673 (strong, -C=C- stretching), 1655 (moderately strong, stretching vibration), 1220 (medium strength, Ar-O-C-asymmetric stretc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com