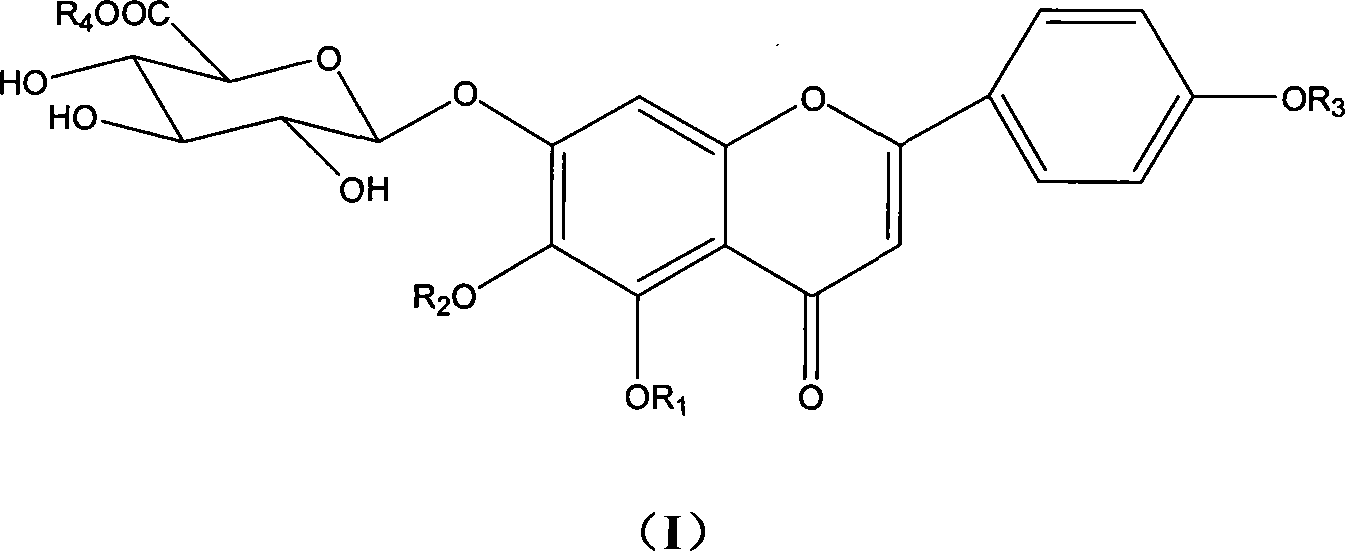
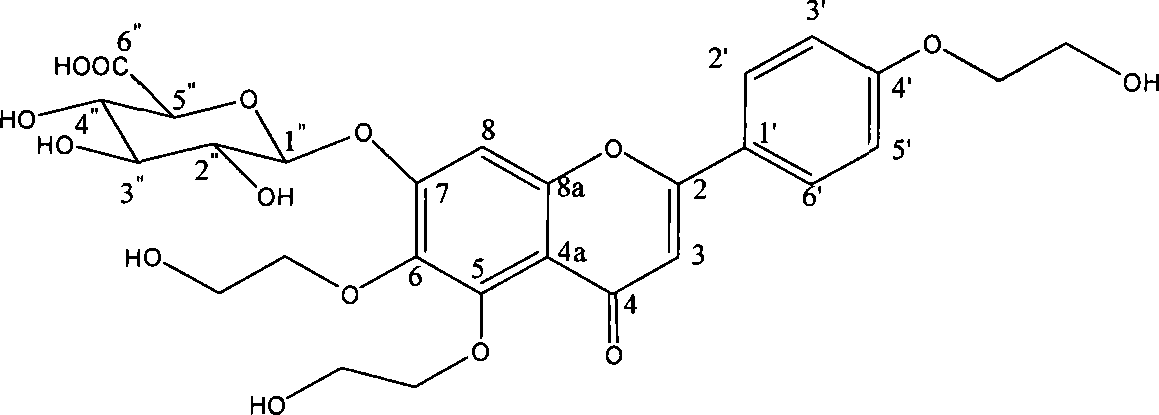
Novel scutellarin compounds and uses thereof
A technology of scutellarin and compounds, which is applied in the field of medicine, can solve the problems of reducing the efficacy of scutellarin, many adverse reactions, and poor water solubility of scutellarin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: The preparation method of 4'-hydroxyethyl scutellarin
[0022] At 50°C, 10g of scutellarin, 10ml of water, and 0.86g of sodium hydroxide were added to the reaction flask, and nitrogen was introduced to remove oxygen; then, ethylene oxide was introduced to react for 5 hours. Cool, filter, and concentrate the filtrate under reduced pressure to obtain the crude product of hydroxyethyl scutellarin. Dissolve the crude product of hydroxyethyl scutellarin in water, absorb it through a macroporous adsorption resin chromatographic column, and elute it with water, collect the water-eluted part, check it by thin-layer chromatography, combine the same fractions, concentrate to dryness under reduced pressure, and wash with ethanol Dissolve, add activated carbon to decolorize, and recover the solvent. The content of 4'-hydroxyethyl scutellarin detected by HPLC was 75%.
Embodiment 2
[0023] Embodiment 2: the method for preparing 6-hydroxyethyl scutellarin
[0024] At 60°C, 10g of scutellarin, 10ml of water, and 0.9g of sodium hydroxide were added to the reaction flask, and nitrogen was introduced to remove oxygen; then, ethylene oxide was introduced to react for 5 hours. Cool, filter, and concentrate the filtrate under reduced pressure to obtain the crude product of hydroxyethyl scutellarin. Dissolve the crude product of hydroxyethyl scutellarin in water, absorb it through a macroporous adsorption resin chromatographic column, and elute it with water, collect the water-eluted part, check it by thin-layer chromatography, combine the same fractions, concentrate to dryness under reduced pressure, and wash with ethanol Dissolve, add activated carbon to decolorize, and recover the solvent. The content of 6-hydroxyethyl scutellarin detected by HPLC was 70%.
Embodiment 3
[0025] Embodiment 3: the method for preparing 4', 6-dihydroxyethyl scutellarin
[0026] At 65°C, add 10g of scutellarin, 10ml of water, and 1.6g of sodium hydroxide into the reaction flask, pass through nitrogen to remove oxygen; then pass through ethylene oxide, and react for 8 hours. Cool, filter, and concentrate the filtrate under reduced pressure to obtain the crude product of hydroxyethyl scutellarin. Dissolve the crude product of hydroxyethyl scutellarin in water, absorb it through a macroporous adsorption resin chromatographic column, and elute it with water, collect the water-eluted part, check it by thin-layer chromatography, combine the same fractions, concentrate to dryness under reduced pressure, and wash with ethanol Dissolve, add activated carbon to decolorize, and recover the solvent. The content of 4', 6-hydroxyethyl scutellarin detected by HPLC was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com