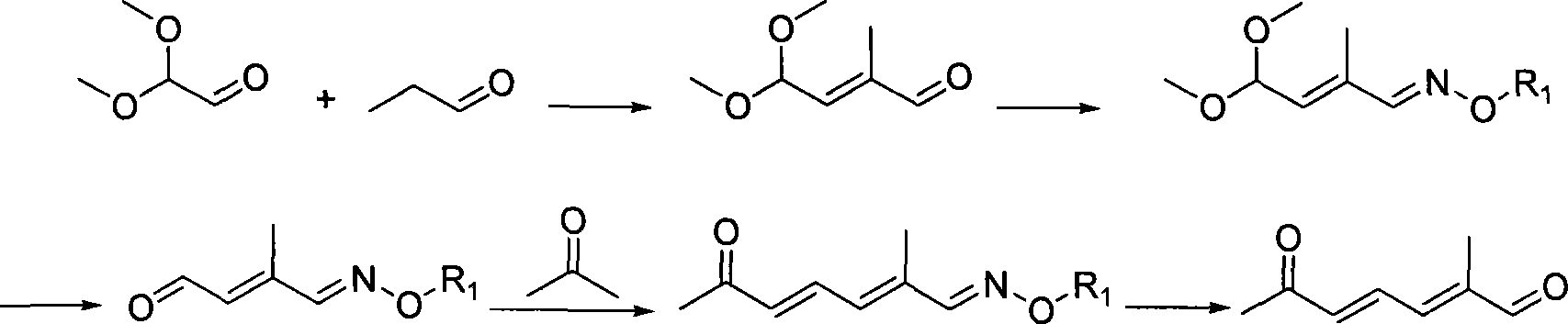
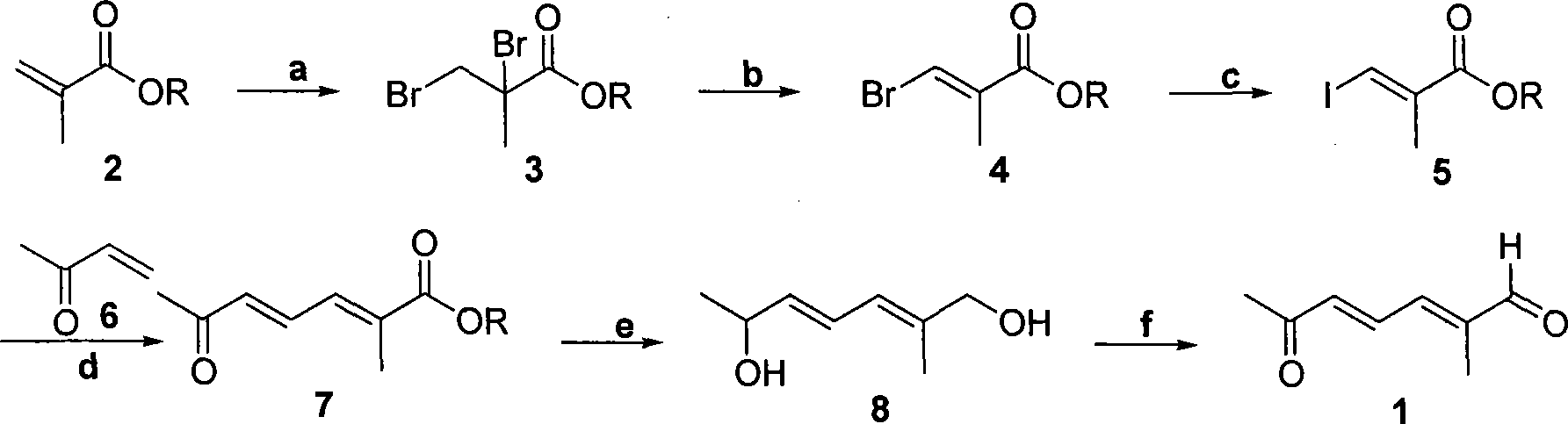
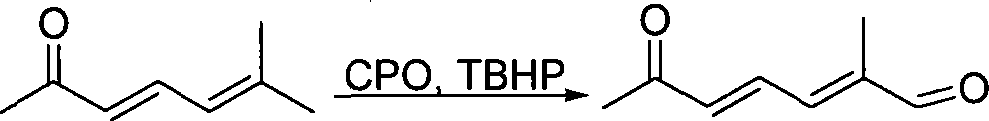Method for preparing (2E,4E)-2-methyl-6-oxo-2,4-heptadienal
A technology of heptadiene aldehyde and heptadiene, which is applied in the field of preparation of organic compounds, can solve problems such as low yield, difficult separation of epoxy compounds, and failure to obtain products, and achieve high reaction yield, easy purification, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] An example of the present invention is provided below.
[0030] Step a.
[0031] Weigh 20 g of 2-methyl methacrylate and dissolve in 20 ml of CCl 4 After stirring well, slowly drop 10.12 ml of liquid bromine (Br 2 ). After dropping, the stirring reaction was continued for 2 hours. The reaction solution was directly used in the next reaction without treatment.
[0032] Reagent and consumption also can obtain similar result in the following ranges in the reaction:
[0033] 1. 2-Methyl methacrylate concentration: 0.6~1.5g / mL;
[0034] 2.Br 2 Molar ratio to 2-methyl methacrylate: 1.0-1.2 is the best;
[0035] 3. Use Br 2 CCl 4 The reaction was carried out using a solution instead of liquid bromine.
[0036] Step b.
[0037] Under cooling in an ice-water bath, slowly drop 45.75 milliliters of DBU (1,8-diazabicyclo[5,4,0]undec-7-ene) into the reaction solution in step a, the solution turns from yellowish brown to milky Pale yellow, a lot of solids precipitated, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com