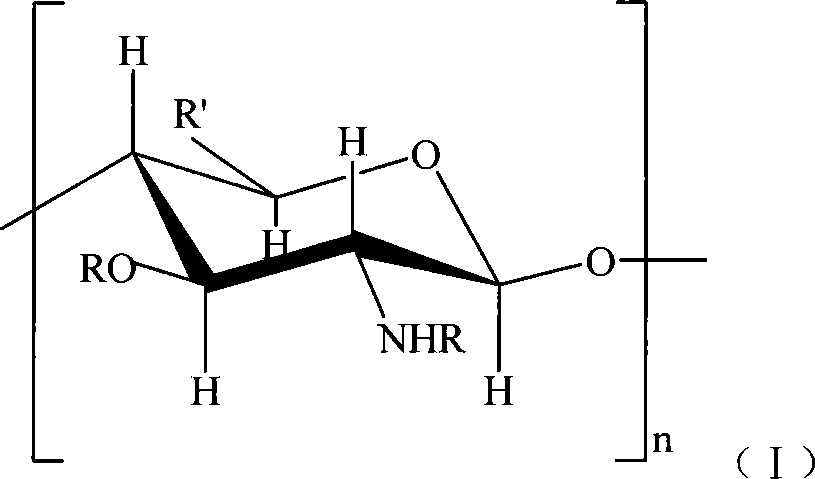
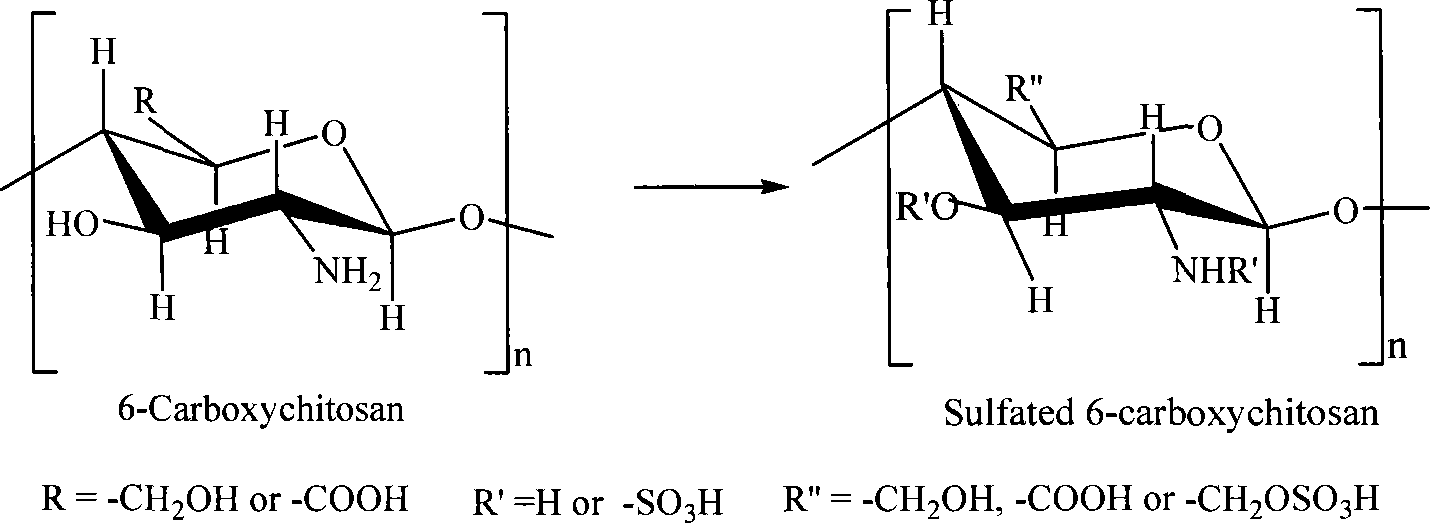
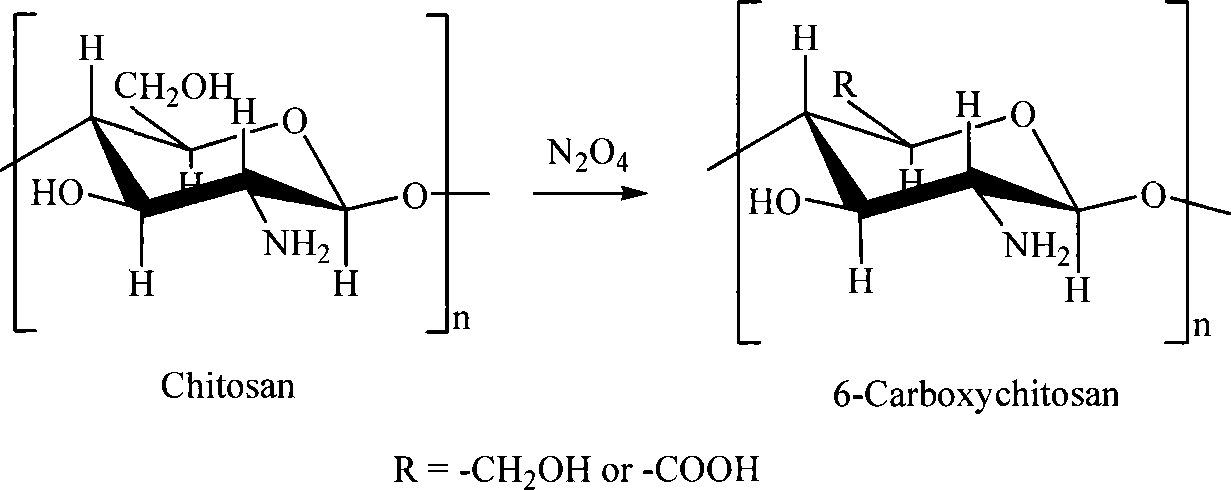Sulfonated acylation 6-carboxyl chitosan as well as salt and preparation method thereof
A technology of carboxyl chitosan and sulfonated acyl, which is applied in the field of chitosan derivatives, can solve the problems of complicated preparation process, poor reaction effect, difficult to remove ions, etc., and achieve strong biocompatibility and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The following reference examples can illustrate the present invention in more detail, but do not limit the present invention in any form.
[0026] Reference Example 1: Preparation of Sulfonated Acetyl 6-Carboxyl Chitosan
[0027] 1. Take 10g of chitosan powder and disperse it in 200mL of glacial acetic acid. After soaking for 24 hours, slowly introduce dry NO into it while stirring. 2 Gas 1.0L, to ensure that the system is always saturated with nitrogen dioxide gas and maintained for 6 hours, centrifuged or separated after adding absolute ethanol or acetone, washed with absolute ethanol and dried to obtain 6-carboxychitosan; the dried 6 -Carboxychitosan powder is dispersed in 100mL glacial acetic acid, after fully soaking, add 50mL of chlorosulfonic acid glacial acetic acid solution therein, react at 65°C for 10 hours, separate the product or add dehydrated ethanol or acetone thereto to separate it, and use Obtain the sulfonated acetyl 6-carboxy chitosan of 9.5g after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com