Peperphentonamine hydrochloride freeze-dried injection and preparation and application thereof
A technology for piperphentonamine hydrochloride and freeze-dried powder injection, which is applied in the field of piperphentonamine hydrochloride freeze-dried powder injection and its preparation, can solve the problems of difficult control, high requirements for freeze-drying equipment, large temperature range, etc. Character difference, ideal freeze-drying effect, good product forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
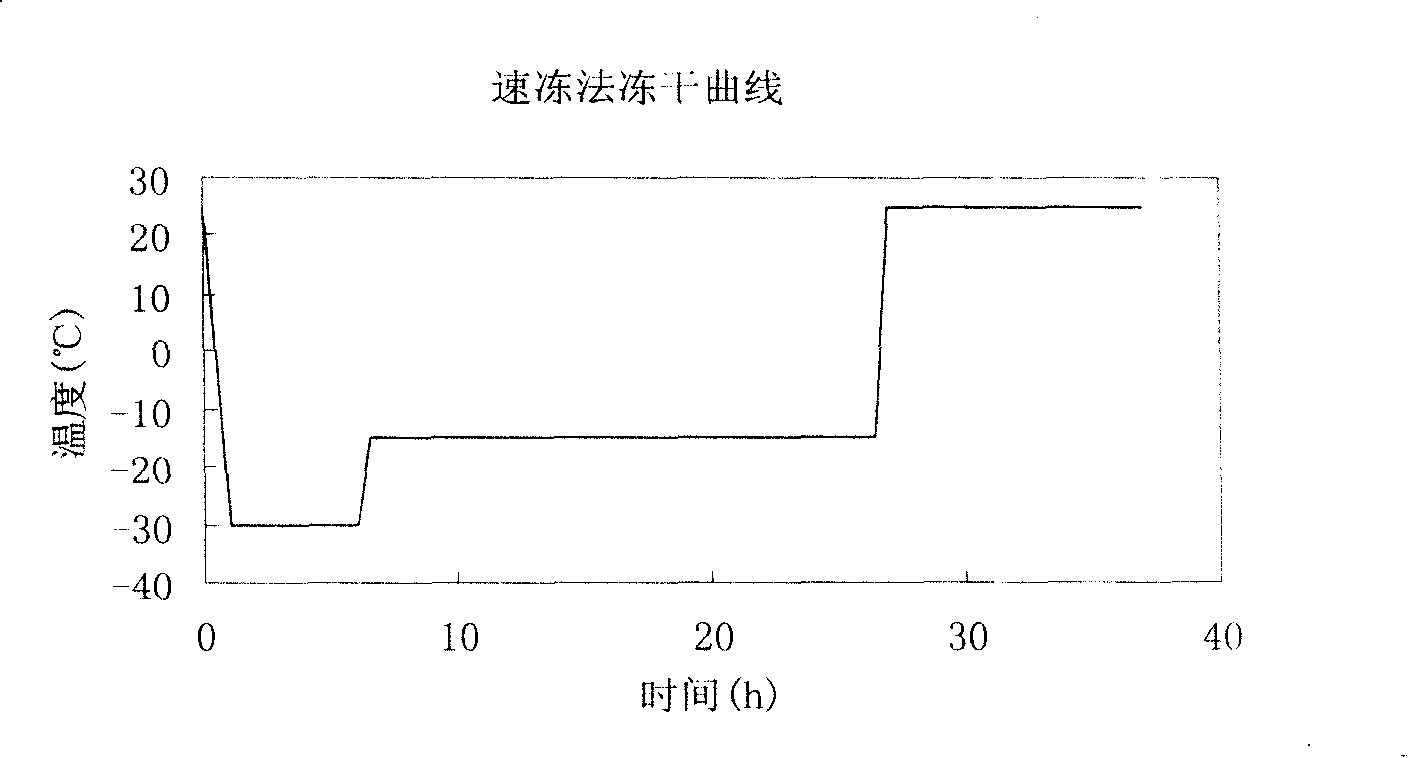
[0040] Weigh piperphentonamine hydrochloride raw material 1.0g and mannitol raw material as excipient 2.5g, add in the flask and add the water for injection 400ml of pH1.5 and mix well, the pH value of the water for injection that the pH value is equal to 1.5 is obtained by means of 1N hydrochloric acid solution for adjustment. Raise the temperature to 40°C, perform ultrasonic treatment, and after all the raw materials are dissolved (about 10 minutes), filter and sterilize with a stainless steel filter under positive pressure, pack in aliquots, 5ml per bottle, and use a half-cap freeze-drying special rubber stopper, first reduce the temperature of the sample chamber to - 25°C, put the sample into pre-freezing for 3 hours, raise the temperature to -20°C for sublimation drying for 15 hours, and then raise the temperature to 20°C for 5 hours to dry. The obtained product properties are as follows:
[0041]
Embodiment 2
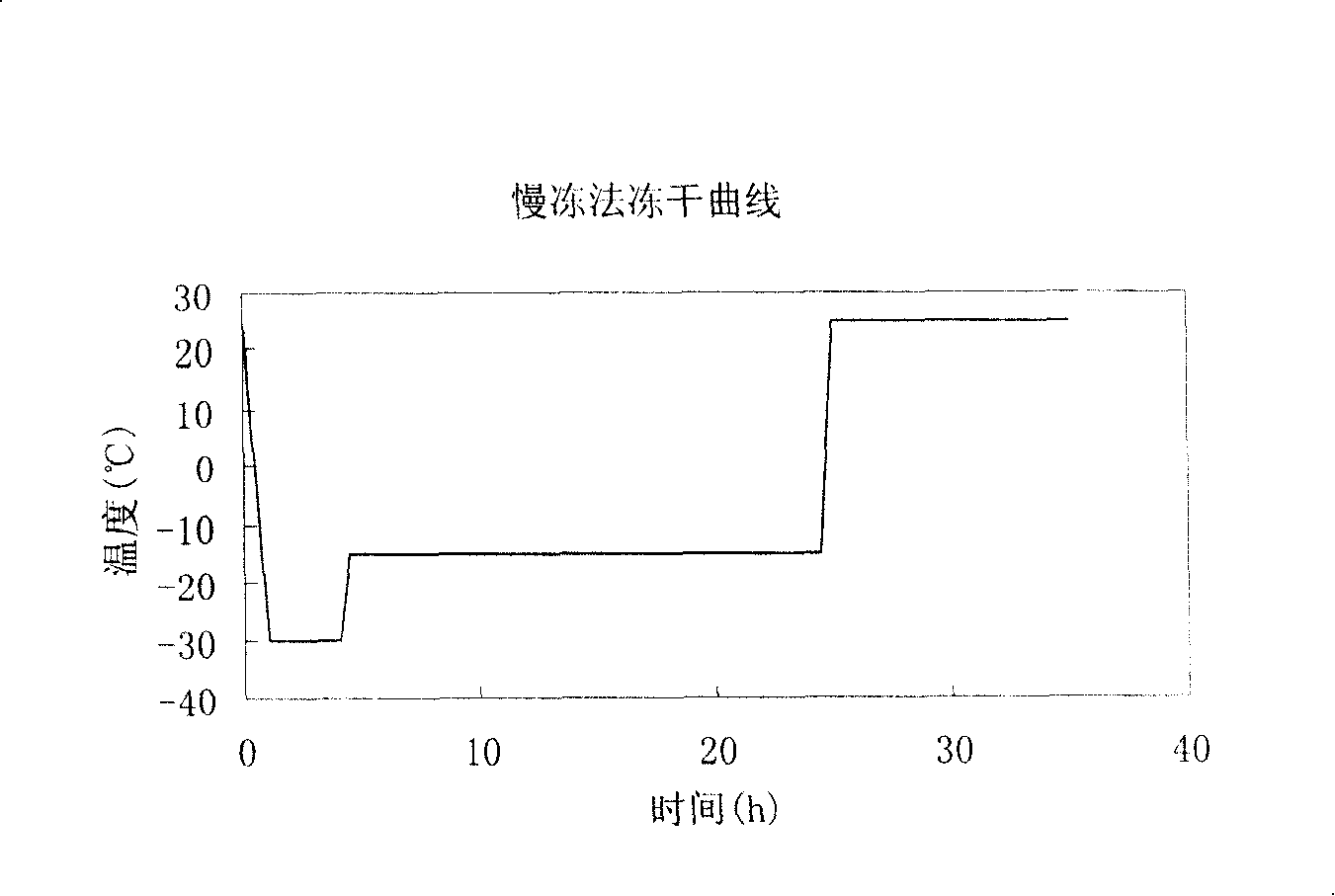
[0043] Weigh 1.0 g of piperphentonamine hydrochloride raw material and 10.0 g of mannitol raw material as excipients, add to the flask and add 500 ml of water for injection with pH 2.0 and mix well, and the water for injection with pH value equal to 2.0 is obtained by means of 1N hydrochloric acid solution adjusted. Raise the temperature to 50°C and perform ultrasonic treatment. After all the raw materials are dissolved (about 10 minutes), the stainless steel filter is positively pressure filtered and sterilized, and divided into 5ml bottles with a half-cap freeze-drying special rubber stopper. First, the temperature of the sample chamber is reduced to - 30°C, put the sample into pre-freezing for 5 hours, raise the temperature to -20°C for sublimation drying for 20 hours, and then raise the temperature to 25°C for 10 hours. The obtained product properties are as follows:
[0044]
Embodiment 3
[0046] Weigh 1.0g of piperphentonamine hydrochloride raw material and 20g of mannitol raw material as excipients, add to the flask and add water for injection with pH 2.5 to 600ml and mix well, and the water for injection with pH value equal to 2.5 is obtained by 1N hydrochloric acid solution For adjustment, raise the temperature to 55°C and perform ultrasonic treatment. After all the raw materials are dissolved (about 10 minutes), the stainless steel filter is positively pressure filtered and sterilized, and divided into 5ml bottles with a special rubber stopper for freeze-drying. Put the good sample into the sample chamber, then lower the temperature of the sample chamber to -30°C, pre-freeze for 4 hours, raise the temperature to -20°C for sublimation and dry for 15 hours, then raise the temperature to 20°C and dry for 5 hours, the properties of the obtained product are as follows: forming, Excellent appearance, not deformed by a little force, easy to dissolve, clear, YG1-2. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com