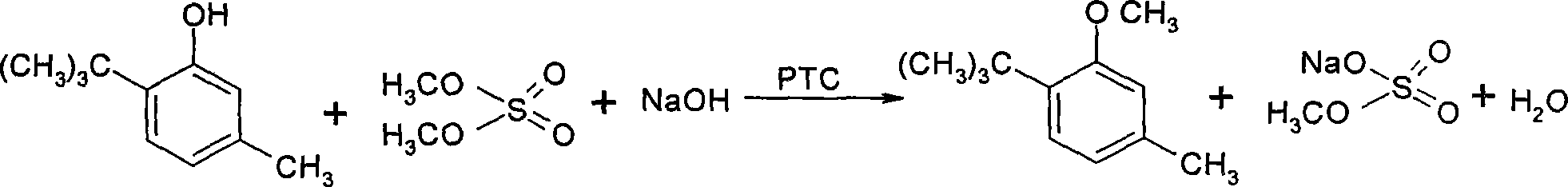
Method for preparing 3-methoxyl-4-t-butyltoluene
A technology of tert-butyltoluene and methoxy, which is applied in the field of organic synthesis in the chemical industry, can solve the problems of not being suitable for industrial production, and achieve the effects of low operating cost, high yield, and easy control of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 153g (0.93mol) content > 99.5% 6-tert-butyl m-cresol, 3.9g ( 12mmol) tetrabutylammonium bromide, after stirring for 1-2 minutes, add 105mL (~139.5g, 1.11mol) dimethyl sulfate. Under ice-water bath cooling and stirring, 109.3 g (1.16 mol) of 42.5% (wt%) sodium hydroxide aqueous solution was added dropwise to the above mixture within 2 to 3 hr. By controlling the dropping rate, the internal temperature during the dropping process was maintained at 35±2°C. After the drop, the above temperature was maintained, and the stirring reaction was continued for 0.5 hr. After the reaction, stop stirring, add 200g of water to the flask, stir and mix for 0.5hr., stop stirring, let stand to separate layers, and separate the water layer. The oil layer was stirred and washed with 300 g of water, and allowed to stand for stratification to obtain 157 g of 3-methoxy-4-tert-butyltoluene. The purity of the product by GC analysis was 99.5%, and the yield was 95%.
Embodiment 2
[0022] Except adopting 3.4g (12.3mol) tetrabutylammonium chloride to replace tetrabutylammonium bromide in the above example, other operations are all the same as in Example 1, thereby obtaining 152g 3-methoxy-4-tert Butyl toluene, GC analysis product purity is 99.2%, yield 91.2%.
Embodiment 3
[0024] Add 407kg (4.324kmol) concentration 42.5% (wt %) sodium hydroxide aqueous solution, 570kg (3.476kmol) content > 99.5% 6-tert-butyl m-cresol and 14.55kg (45.19mol) successively in 2000L stirring reactor ) Tetrabutylammonium bromide, start stirring, feed cold brine into the jacket of the reaction kettle, add 393L (522kg, 4.14kmol) sulfuric acid dropwise to the above mixture from the dimethyl sulfate metering tank within 6 to 8hr. Dimethyl ester, by controlling the rate of addition of dimethyl sulfate and the flow of cold brine, the temperature of the material in the reactor is maintained at 36-38°C. After dropping dimethyl sulfate (in the above reaction, the mol ratio of feeding is: 6-tert-butyl m-cresol: dimethyl sulfate: sodium hydroxide: tetrabutylammonium bromide=1: 1.192: 1.244: 0.013 (mol:mol)), maintain the above temperature and continue to stir the reaction for 1hr. After the reaction, add 600L of primary water to the reaction kettle, stir and mix for 0.5hr., let...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com