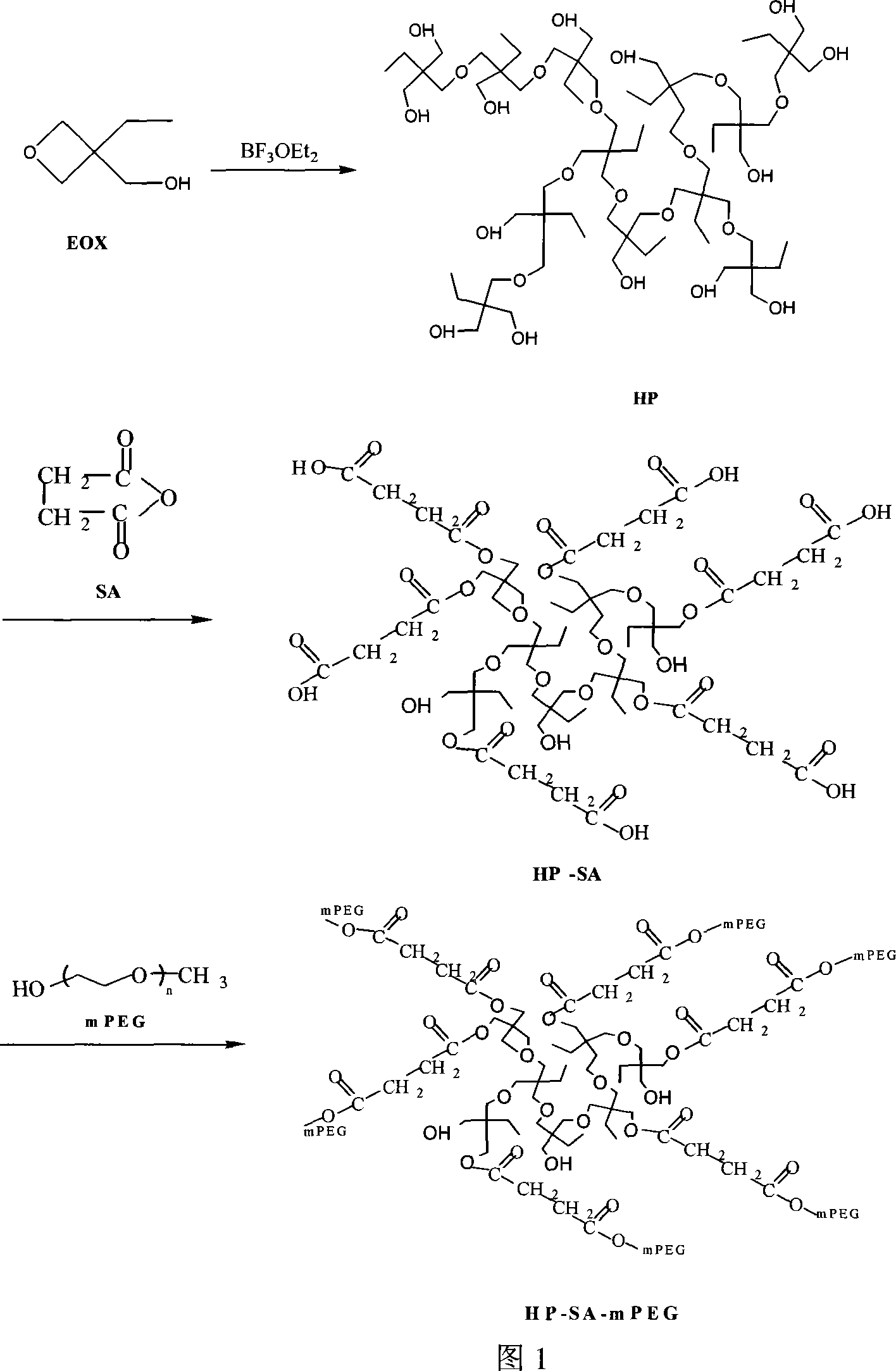
Degradable polyglycol modified ultra-branching polyether ester and preparation method thereof
A polyethylene glycol modified and hyperbranched polyether technology, which is applied in the field of medical materials, can solve the problems of biocompatibility reduction and achieve the effect of accelerating the degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] This embodiment 1 is implemented under the following conditions of implementation and technical requirements:
[0023] a In a four-necked flask equipped with an electromagnetic stirrer, a thermometer, a nitrogen inlet and an addition funnel, first pass nitrogen for 30 minutes to remove the air in the reaction system. 7.096g (0.05mol) boron trifluoride ether BF 3 .OEt 2 and 80ml of dichloromethane were added into the flask, and the temperature of the control system was 10°C. Within 5 minutes, 11.62g (0.1mol) of the monomer 3-ethyl-3-butoxanemethanol was slowly added dropwise to the reaction system to maintain The temperature, the reaction 48h. The reaction solution was slowly added dropwise to excess anhydrous diethyl ether, a white precipitate was produced, filtered, washed, and vacuum-dried at 100°C until constant weight. The productive rate of gained hyperbranched product is 92%, and degree of branching is 0.41 (because 13 C-NMR characterizes the degree of branchi...
Embodiment 2
[0026] This embodiment 2 is implemented under the following conditions of implementation and technical requirements:
[0027] a In a four-necked flask equipped with an electromagnetic stirrer, a thermometer, a nitrogen inlet and an addition funnel, first pass nitrogen for 30 minutes to remove the air in the reaction system. Add 7.096g (0.05mol) of boron trifluoride ether BF3.OEt2 and 80ml of chloroform into the flask, control the temperature of the system at 30°C, and within 5 minutes, 11.62g (0.1mol) of monomer 3-ethyl-3 -Butoxanemethanol was slowly added dropwise into the reaction system, and the temperature was maintained for 24 hours of reaction. The reaction solution was slowly added dropwise to excess anhydrous diethyl ether, a white precipitate was produced, filtered, washed, and vacuum-dried at 100°C until constant weight. The yield of the obtained hyperbranched product was 83.5%, and the degree of branching was 0.47.
[0028] b After dissolving the product obtained ...
Embodiment 3
[0030] This embodiment 3 is implemented under the following conditions of implementation and technical requirements:
[0031] a In a four-necked flask equipped with an electromagnetic stirrer, a thermometer, a nitrogen inlet and an addition funnel, first pass nitrogen for 30 minutes to remove the air in the reaction system. Add 7.096g (0.05mol) boron trifluoride ethyl ether BF3.OEt2 and 80ml xylene to the flask, control the system temperature to 70°C, and within 5 minutes, 11.62g (0.1mol) monomer 3-ethyl-3- Butoxymethanol was slowly added dropwise into the reaction system, and the temperature was maintained for 96 hours of reaction. The reaction solution was slowly added dropwise to excess anhydrous diethyl ether, a white precipitate was produced, filtered, washed, and vacuum-dried at 100°C until constant weight. The yield of the obtained hyperbranched product was 94.5%, and the degree of branching was 0.55.
[0032]b Dissolve the product obtained in a with toluene, react 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com