Catalytic oxidation technique for calcium sulfite
A technology for catalytic oxidation and calcium sulfite, applied in chemical instruments and methods, separation methods, separation of dispersed particles, etc., can solve problems such as unsuitable limestone-gypsum technology, achieve significant catalytic oxidation of calcium sulfite, reduce system The possibility of fouling, the effect of facilitating desulfurization operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
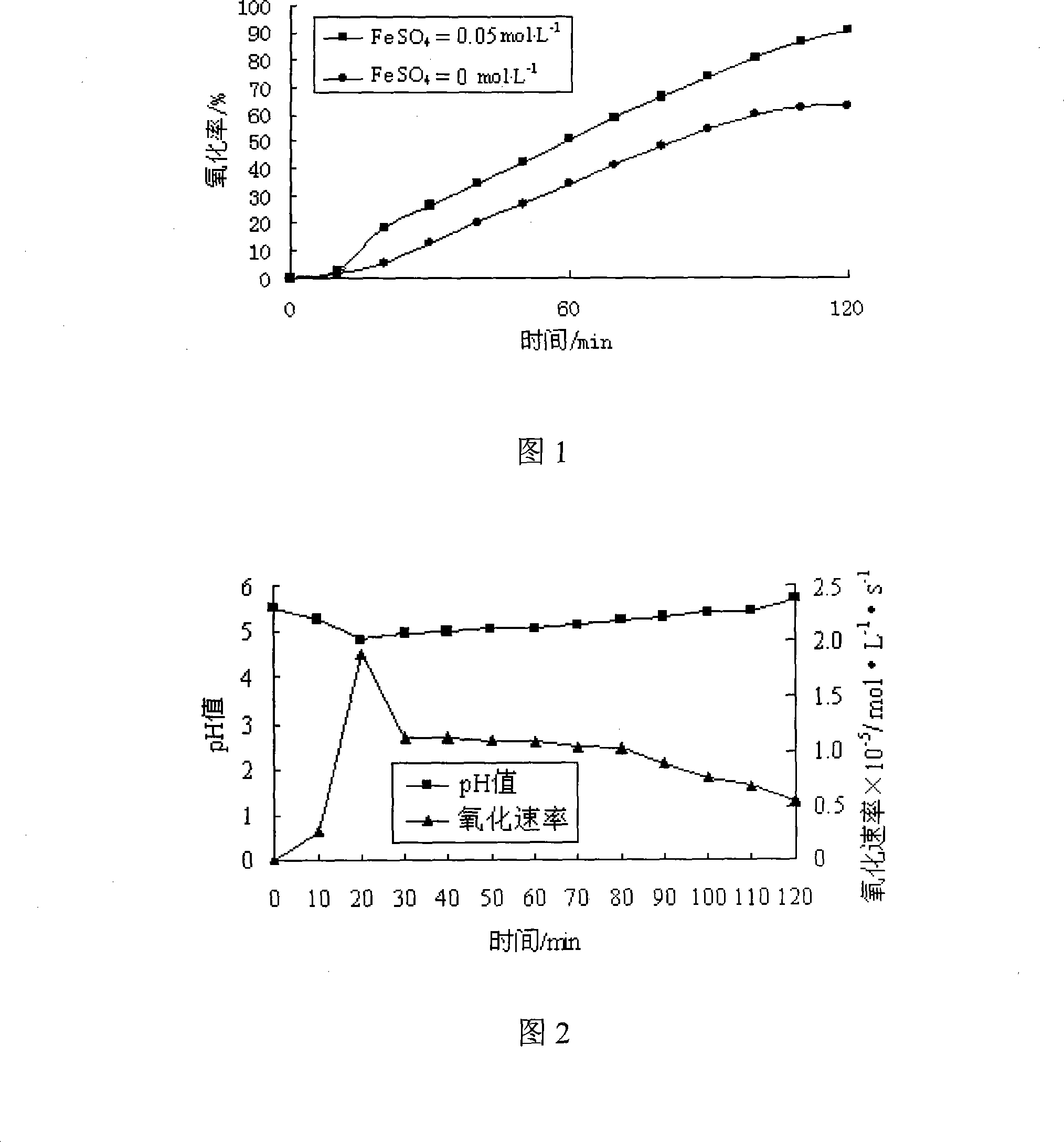
[0017] Add FeSO4 solution with a concentration of 0.05mol / L in the calcium sulfite slurry, keep the pH value at about 5-6, and carry out forced oxidation on the slurry. The curve of the oxidation rate over time is shown in Figure 1. It is found that when the concentration of FeSO4 catalyst is 0.05mol / L, the oxidation of calcium sulfite is 91.2%, while the oxidation process without catalyst is only 63.4%. In addition, it can also be seen from Figure 2 that when the catalyst is added, the pH value can be kept in a relatively stable state, and the oxidation rate is also in a relatively high range.
Embodiment 2
[0019] Add a MnSO4 solution with a concentration of 0.05mol / L to the calcium sulfite slurry, keep the pH value at about 5-6, carry out forced oxidation on the slurry, and make the relationship diagram of oxidation rate and time. It can also be seen that when MnSO4 is added After the catalyst, the oxidation rate of calcium sulfite is only 63.4%. In addition, it can also be seen from the relationship diagram of pH value, oxidation rate and time in the catalytic oxidation process that when the catalyst is added, the pH value can be kept in a relatively stable state, and the oxidation rate is also in a relatively high range.
Embodiment 3
[0021] Add MnSO4 and FeSO4 solutions with a concentration of 0.05mol / L to the calcium sulfite slurry. There is no special requirement for the ratio between the two, but the pH value of the slurry should be kept at about 5-6 after adding. It can also be seen from the graph of the relationship between the oxidation rate and time that when the MnSO4 catalyst is added, the oxidation rate of calcium sulfite increases significantly, the pH value can be kept in a relatively stable state, and the oxidation rate is also in a relatively high range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com