Preparation of hydrogen-storage material used for fuel cell
A technology of hydrogen storage materials and fuel cells, which is applied in fuel cells, circuits, electrical components, etc., can solve the problems of difficult control of reaction speed, loss of the role of hydrophilic materials in guiding fluid, and hindrance of further reaction of solid hydrides, etc., to achieve Reduce the amount of addition, easy to operate, and improve the effect of conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
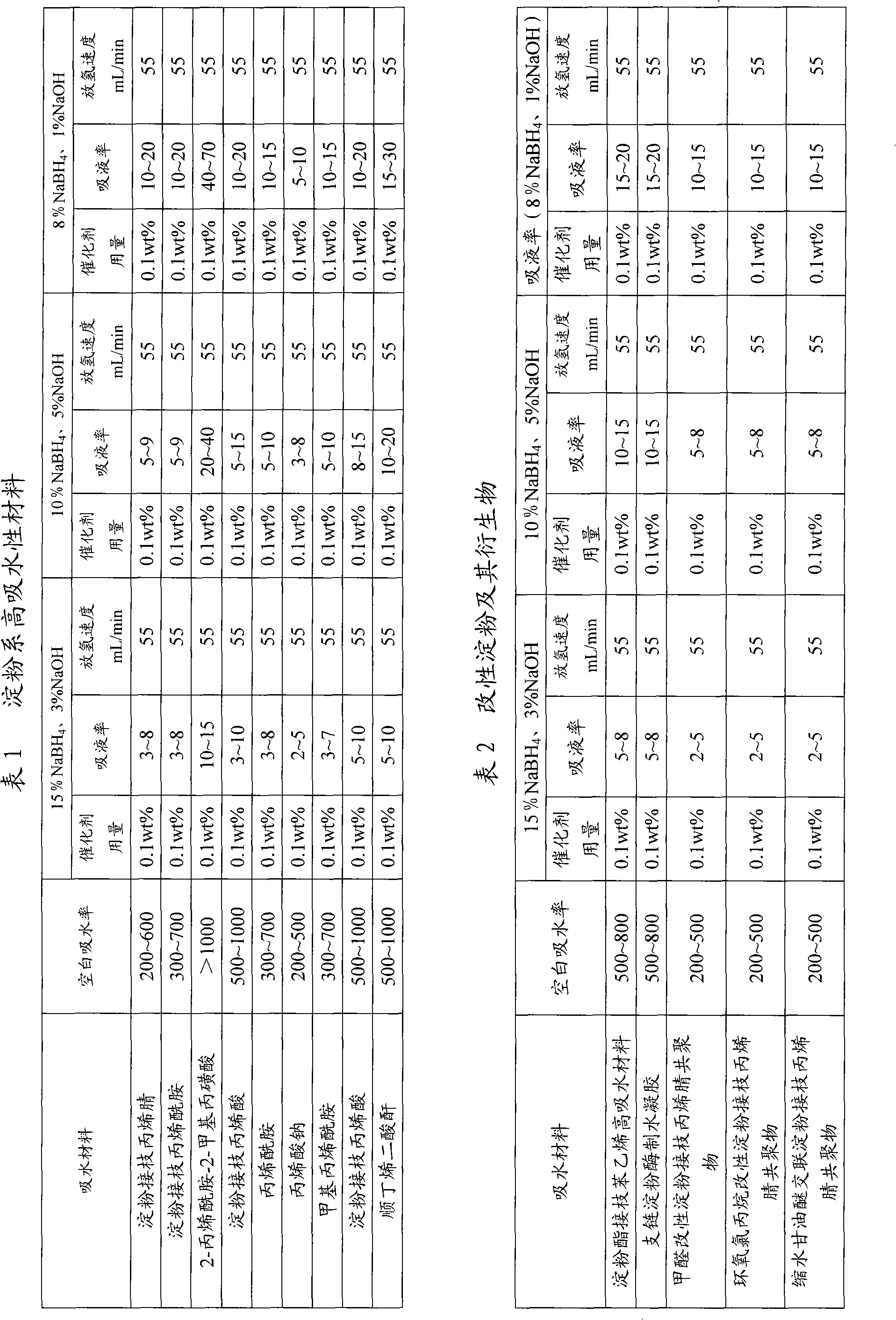
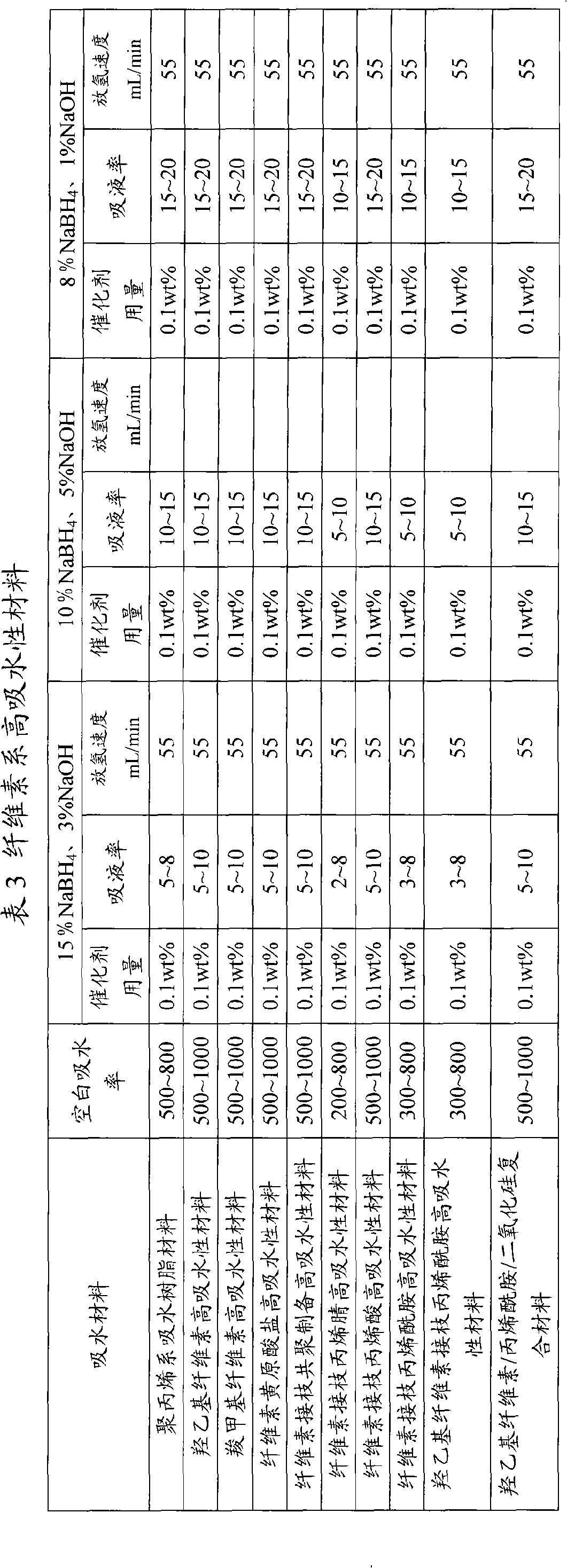
Image
Examples
Embodiment 1
[0025]Hydrogen storage material: sodium borohydride 100g, sodium hydroxide 38g, hydroxyethyl cellulose superabsorbent material 22g, catalyst potassium chloroplatinate K 2 PtCl 6 4.5g;
[0026] Preparation method: Sodium borohydride, sodium hydroxide, hydroxyethyl cellulose superabsorbent material, catalyst potassium chloroplatinate K 2 PtCl 6 well mixed;
[0027] The method for preparing hydrogen using the hydrogen storage material: inject 500g of water into the hydrogen storage material, sodium borohydride and sodium hydroxide dissolve to form an alkaline solution, and the water-absorbing material absorbs the alkaline solution to form a gel-like hydrogen storage material; catalyst Potassium chloroplatinate K 2 PtCl 6 It is reduced to metal platinum by sodium borohydride and evenly distributed in the alkaline solution of sodium borohydride. Under the action of metal platinum, sodium borohydride is hydrolyzed to produce hydrogen.
Embodiment 2
[0029] Hydrogen storage material: sodium borohydride 100g, sodium hydroxide 0.01g, cellulose xanthate superabsorbent material 25g, catalyst nickel powder 30g;
[0030] Preparation method: uniformly mix sodium borohydride, sodium hydroxide, cellulose xanthate superabsorbent material, and catalyst nickel powder to obtain hydrogen storage material;
[0031] The method for preparing hydrogen using the hydrogen storage material: inject 500g of water into the hydrogen storage material, sodium borohydride and sodium hydroxide dissolve to form an alkaline solution, and the water-absorbing material absorbs the alkaline solution to form a gel-like hydrogen storage material; catalyst Evenly distributed in the alkaline solution of sodium borohydride, under the action of the catalyst, the sodium borohydride is hydrolyzed to produce hydrogen.
Embodiment 3
[0033] Hydrogen storage material: Potassium borohydride 100g, sodium hydroxide 2g, cellulose grafted acrylamide super absorbent material 0.1g, catalyst cobalt powder 45g;
[0034] Preparation method: Mix potassium borohydride, sodium hydroxide, cellulose-grafted acrylamide superabsorbent material, and catalyst cobalt powder evenly to obtain hydrogen storage material;
[0035] The method for preparing hydrogen by using the hydrogen storage material: injecting 500g of water into the hydrogen storage material, dissolving potassium borohydride and sodium hydroxide to form an alkaline solution, and the water-absorbing material absorbs the alkaline solution to form a gel-like hydrogen storage material; The catalyst is evenly distributed in the alkaline solution of potassium borohydride, and under the action of the catalyst, the potassium borohydride is hydrolyzed to generate hydrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com