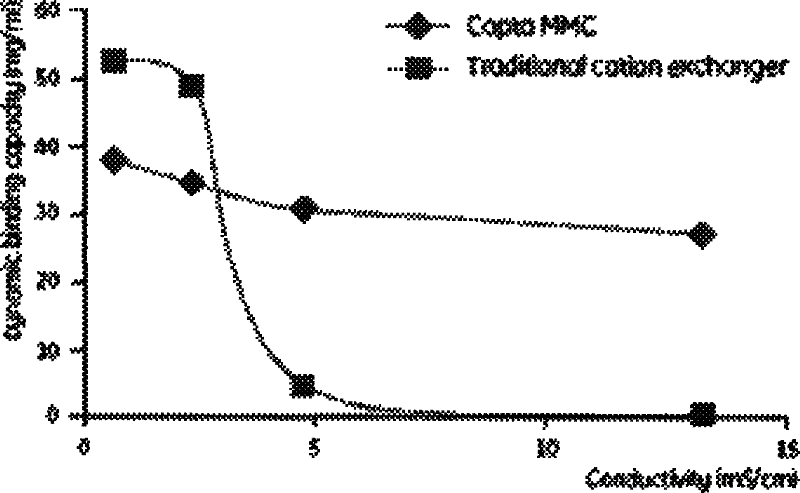
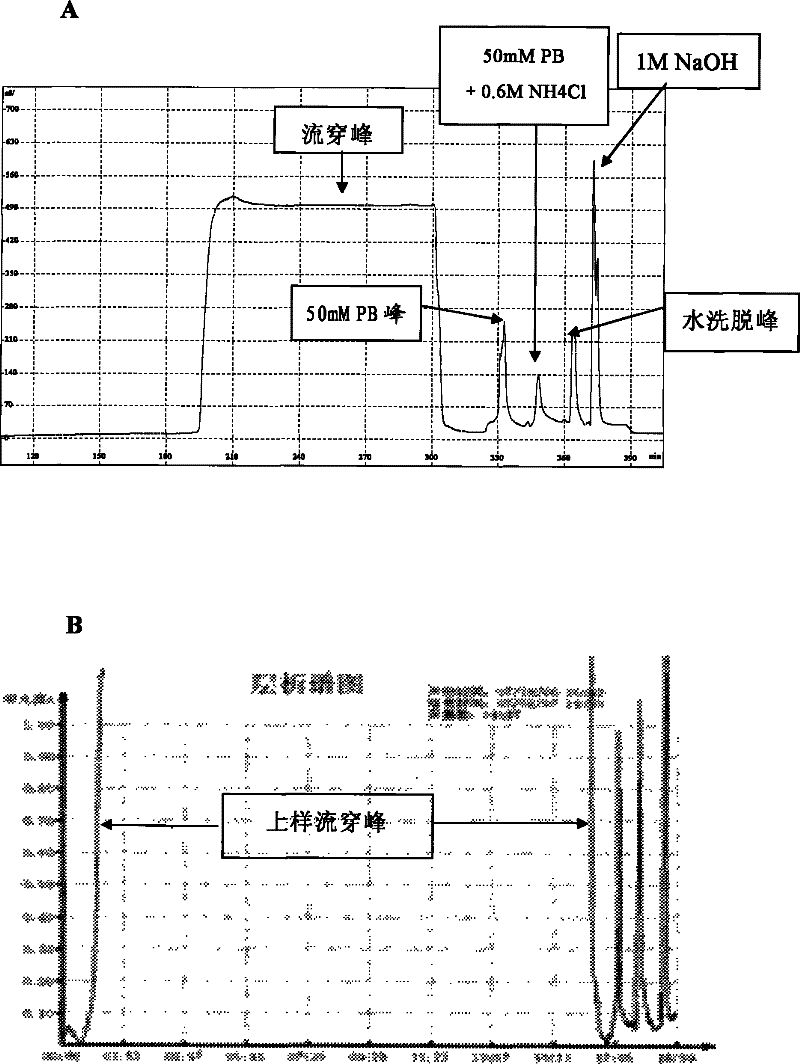
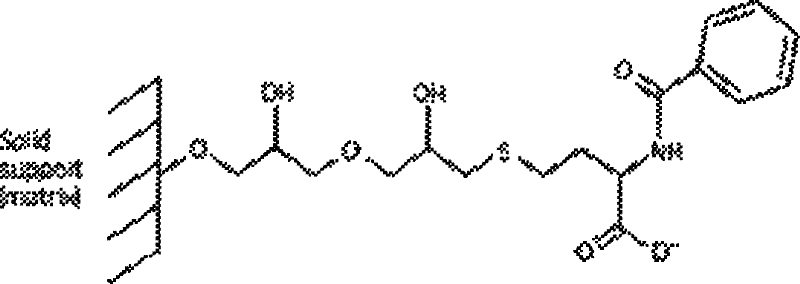Technique for separating and purifying recombination human serum albumin and fusion protein thereof
A technology of human serum albumin and fusion protein, which is applied in the field of purification of human serum albumin and human serum albumin fusion protein, can solve the problems of not being able to purchase and trial, and not mentioning the purification of human serum albumin fusion protein, etc. Achieve the effect of simple purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: High-density fermentation of engineering strains expressing rHSA and its fusion protein Pichia pastoris
[0038]The recombinant human serum albumin engineering expression bacteria used in the present invention, the construction of fusion protein engineering expression bacteria formed by various human serum albumin and therapeutic proteins directly or through linking peptides are all obtained from the Chinese authorized invention patent ZL02, ZL2004100, ZL2007100 and US authorized invention patent US7244 and US invention patent application US2006.
[0039] The rHSA / therapeutic protein fusion protein strain involved therein can utilize the same production process to obtain the expression product. It is generally described as, 1ml of the frozen strain of Pichia pastoris high-expression engineered bacteria is inoculated in 100ml of inorganic salt medium, and cultured at 30°C for 20 hours at 300rpm to reach OD 600 =~11. The average inoculation in five 200ml inor...
Embodiment 2
[0040] Example 2: Vertebrate cells are used to express human serum albumin / EPO fusion protein
[0041] Recombinant cell clones obtained by transfecting adherent CHO cells (CHO-H) or CHO suspension cells (Invitrogen CHO-S cell line) with pCMV-HSA or pCMV-HSA / EPO plasmids, using Neomycin as selection pressure The progeny were cultivated and amplified with serum-free medium (commercially available). In the production stage, 3 days after cell inoculation, continuous culture is started, and serum-free culture supernatant containing human serum albumin or human serum albumin EPO cell culture is collected. At this time, the content of the recombinant protein product in the cell culture medium is above 90mg / L, the pH=7.0, and the conductivity value is between 5-20ms / cm.
Embodiment 3
[0042] Embodiment 3: yeast high-density fermentation post-treatment
[0043] Yeast high-density fermentation broth is pumped into the plate-and-frame filtration system to remove the bacteria to obtain a clarified supernatant. The supernatant can be directly used for separation and purification procedures after suction filtration through a 0.45uM tube filter. The fermentation broth can also be added with active carbon to 0.4-5% after the plate and frame filtration is completed. After mixing well, the activated carbon was removed by a continuous flow high-speed tubular centrifuge to reduce the pigment content in the fermentation broth. After activated carbon treatment, the fermentation supernatant was filtered through a 0.45uM filter before entering the purification procedure. The culture medium containing the target protein obtained from culturing CHO cells in serum-free medium was processed in a continuous-flow high-speed tubular centrifuge, sterilized by a 0.45uM membrane, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com