Preparation method of amisulpride
An amisulpride and amino technology, which is applied in the field of chemical and chemical raw materials synthesis and preparation, can solve problems such as difficult operation in industrial production, need for wastewater treatment, need to be recycled, etc., and achieves low production cost, low impurity content, and reduced addition amount. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
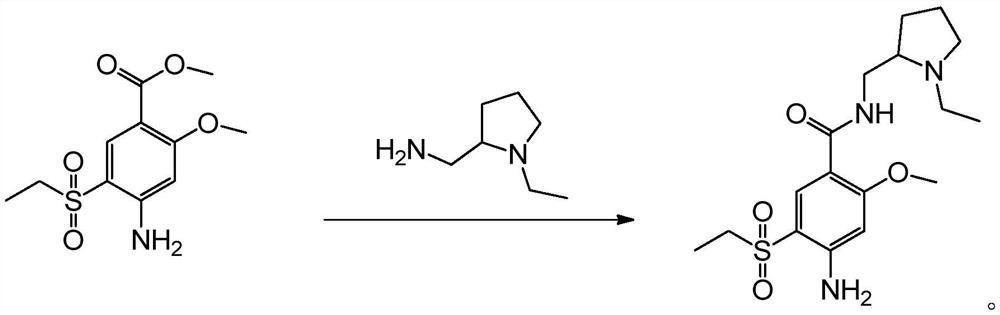
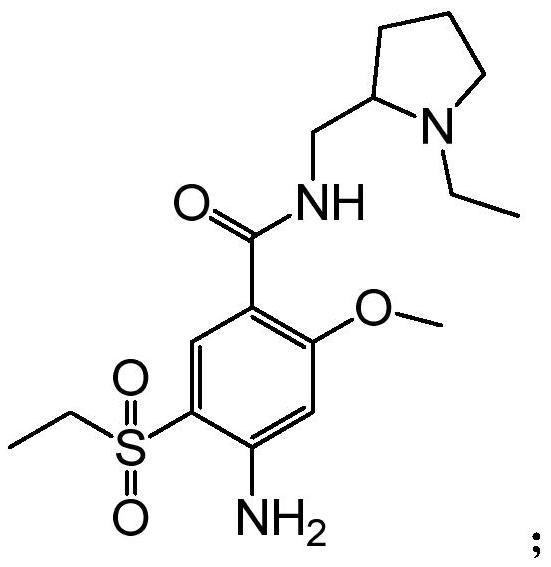
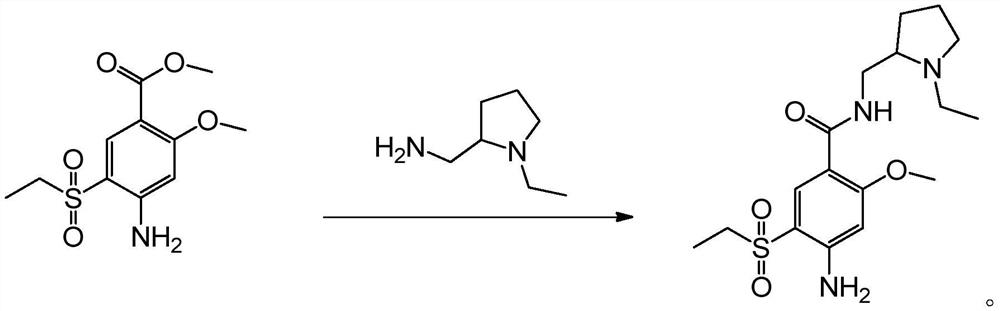
[0021] The present invention provides a kind of preparation method of amisulpride, and concrete synthetic route is as follows:
[0022]
[0023] The principle is: use 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (A-4) as raw material, directly carry out transesterification reaction with N-ethyl-2-aminomethylpyrrole, Get amisulpride. Specifically, it can be divided into two methods for transesterification without adding water and adding water, as follows:
[0024] (Without adding water) Method 1: In an atmospheric distillation device, add 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (A-4) and N-ethyl-2-aminomethyl Base pyrrole, carry out transesterification reaction at 70-90°C, and distill methanol while reacting. When the distillate methanol is no longer distilled out, stop the reaction and carry out vacuum distillation to distill the excess N-ethyl-2-aminomethylpyrrole under reduced pressure at 3-5mmHg pressure and 60-70°C. The N-ethyl-2-amin...
Embodiment 1
[0027] In a 5L three-necked flask, first add 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (A-4) 500g, then add N-ethyl-2-aminomethylpyrrole 2000g ( The mass ratio of 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester to N-ethyl-2-aminomethylpyrrole is 1:4), and the outlet is equipped with an atmospheric distillation device. The transesterification reaction was carried out at 80°C, and the methanol was distilled off during the reaction. After 10 hours, the reaction was stopped when distilled methanol no longer distilled off. Distillation under reduced pressure was carried out again, and excess N-ethyl-2-aminomethylpyrrole was distilled out under reduced pressure at 3 mmHg pressure and 60°C. The N-ethyl-2-aminomethylpyrrole recovered by distillation under reduced pressure can be used in the next reaction. After vacuum distillation, the crude product of amisulpride was obtained in the three-necked flask, and 2000g of acetone was added to obtain the solutio...
Embodiment 2
[0029] In a 100L stainless steel reaction kettle bottle, add 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (A-4) 10kg, then add N-ethyl-2-aminomethylpyrrole 50Kg (The mass ratio of 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester to N-ethyl-2-aminomethylpyrrole is 1:5), and the transesterification reaction was carried out at 70°C, Methanol was distilled off during the reaction. After 12 hours, the reaction was stopped when distilled methanol no longer distilled off. Change to vacuum distillation, and excess N-ethyl-2-aminomethylpyrrole was distilled out under reduced pressure at 5 mmHg pressure and 70°C. The N-ethyl-2-aminomethylpyrrole recovered by distillation under reduced pressure can be used in the next reaction. After distillation under reduced pressure, the crude product of amisulpride was obtained in the reaction kettle, and 40kg of acetone was added to obtain the solution, and then the recrystallization operation was carried out, specifically:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com