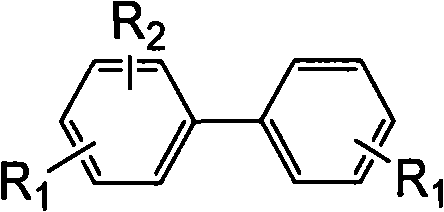
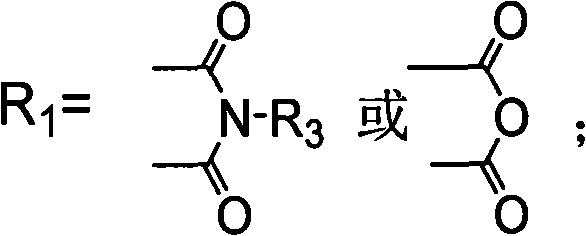
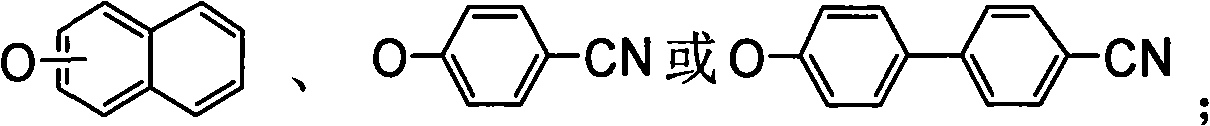Biphenyl compound with dissymmetrical structure, preparation method and application thereof
A compound and asymmetric technology, applied in the direction of organic chemistry, can solve the problems of many by-products, increasing the purity of inorganic sodium ions, complex reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1 contains the synthesis of the I-1 monomer of N-methyl structure
[0077] Add 1.52g (0.038mol) of sodium hydroxide and 4.5g (0.019mol) of N-methyl-4-bromophthalimide into a 100mL three-necked flask, then add 15mL of distilled water, and add 0.225g of palladium carbon , then the temperature was raised to 90° C., a solution of hydroxylamine sulfate (1.53 g, 15 ml of water) was added dropwise, and stirred for 10 hours. After the reaction, hot filtration was obtained to obtain the filtrate, and the pH value was adjusted to 1.5 with an acid solution, and a precipitate was precipitated, which was filtered and dried to obtain the intermediate 1 containing N-methyl structure. Add 2.0 g of intermediate 1 into a 100 mL three-necked flask, add 15 g of concentrated sulfuric acid, heat up to 40°C and stir until homogeneous, add dropwise 0.64 ml of fuming nitric acid, and stir for 6 hours. After the reaction, the reaction solution was poured into a beaker containing 100m...
Embodiment 2
[0086] Embodiment 2 contains the synthesis of the 1-2 monomer of N-methyl structure
[0087] In a 500mL three-necked flask, 23.38g (0.064mol) of I-1 monomer containing N-methyl structure, 250mL of N,N-dimethylformamide (DMF), 10.61g (0.0768mol) of anhydrous potassium carbonate were added successively. ), 5.74g (0.0832mol) of sodium nitrite, stopped after reacting at 130°C for 18 hours in a nitrogen atmosphere. Slowly pour the reaction solution into 1000 mL of hydrochloric acid aqueous solution to precipitate (pH=2-3), stir well, a large amount of yellow precipitate appears, filter the precipitate, and wash with water and air-dry. Recrystallize with distilled water to obtain I-2 monomer containing N-methyl structure.
[0088] I-2 monomer containing N-methyl structure (hydroxyl in 3 position) 1 H-NMR (DMSO-d 6 , ppm):
[0089] 3.14(s, 6H, CH 3 ), 7.37-7.39 (d, 1H, Ar-H), 7.89-7.92 (m, 2H, Ar-H), 8.18-8.20 (d, 1H, Ar-H), 8.34-8.36 (d, 1H, Ar-H) -H), 9.31(s, 1H, OH).
[009...
Embodiment 3
[0096] Embodiment 3 contains the synthesis of the I-3 monomer of N-methyl structure
[0097] Add 3.612g (0.0384mol) of phenol, 5.746g (0.0416mol) of anhydrous potassium carbonate, 11.69g (0.032mol) and DMF60mL of I-1 monomer of N-methyl structure in the 250mL three-necked flask, nitrogen protection, in Stir at 10°C for 24 hours. After the reaction, the reaction solution was poured into water to precipitate, filtered and dried to obtain the I-3 monomer containing N-methyl structure.
[0098] I-3 monomer containing N-methyl structure (phenoxy in 3 position) 1 H-NMR (DMSO-d 6 , ppm):
[0099] 3.16(s, 6H, CH 3 ), 6.92-7.22(m, 5H, Ar-H), 7.56-7.58(d, 1H, Ar-H), 7.90-7.92(dd, 1H, Ar-H), 8.06-8.08(d, 1H, Ar-H) -H), 8.18-8.20(d, 1H, Ar-H), 8.34-8.36(d, 1H, Ar-H).
[0100] Elemental analysis calculated values: C: 69.90, H: 3.91, N: 6.79, O: 19.40.
[0101] Elemental analysis measured values: C: 69.94, H: 3.90, N: 6.77, O: 19.43.
[0102] I-3 monomer containing N-methyl structur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com