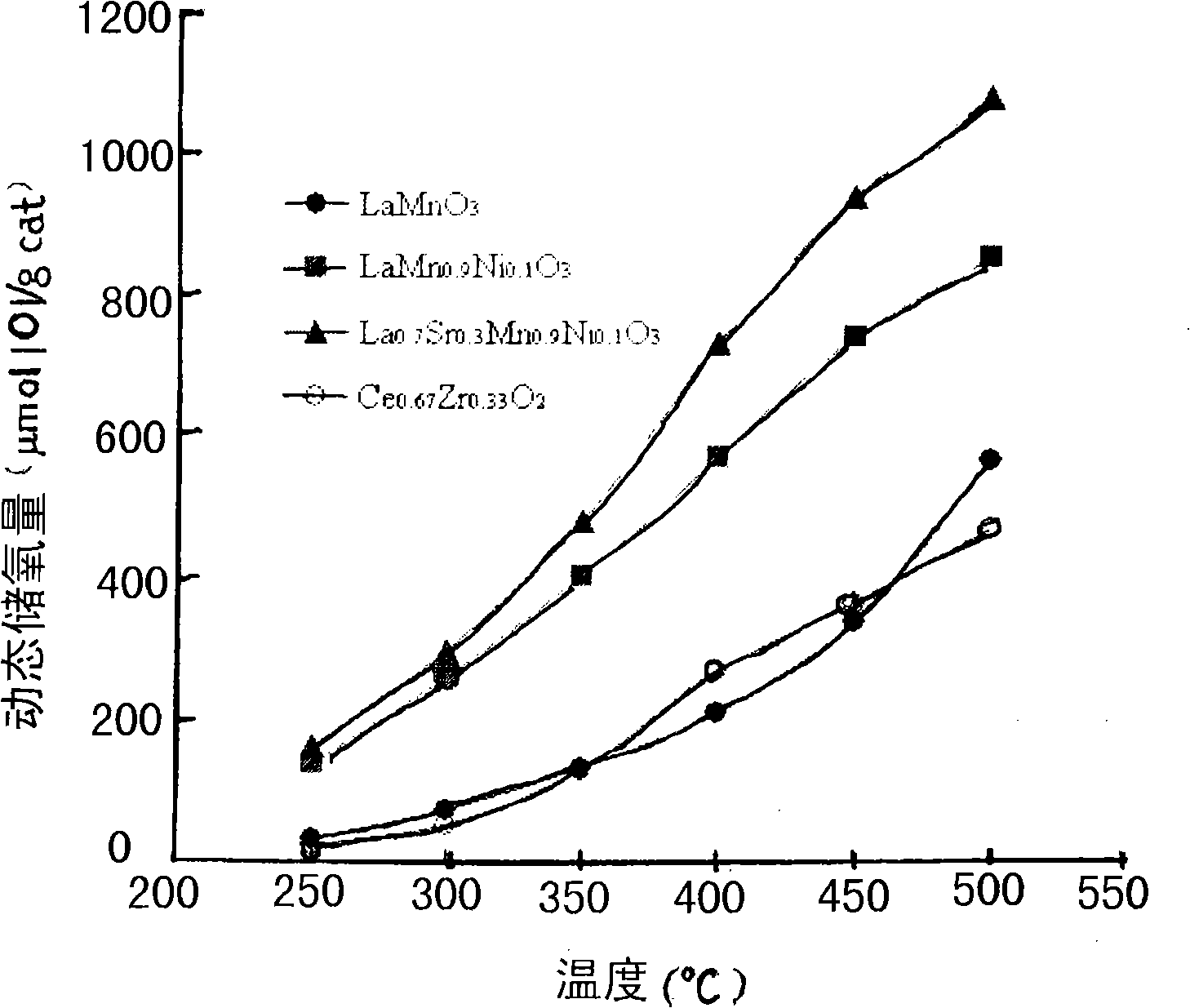
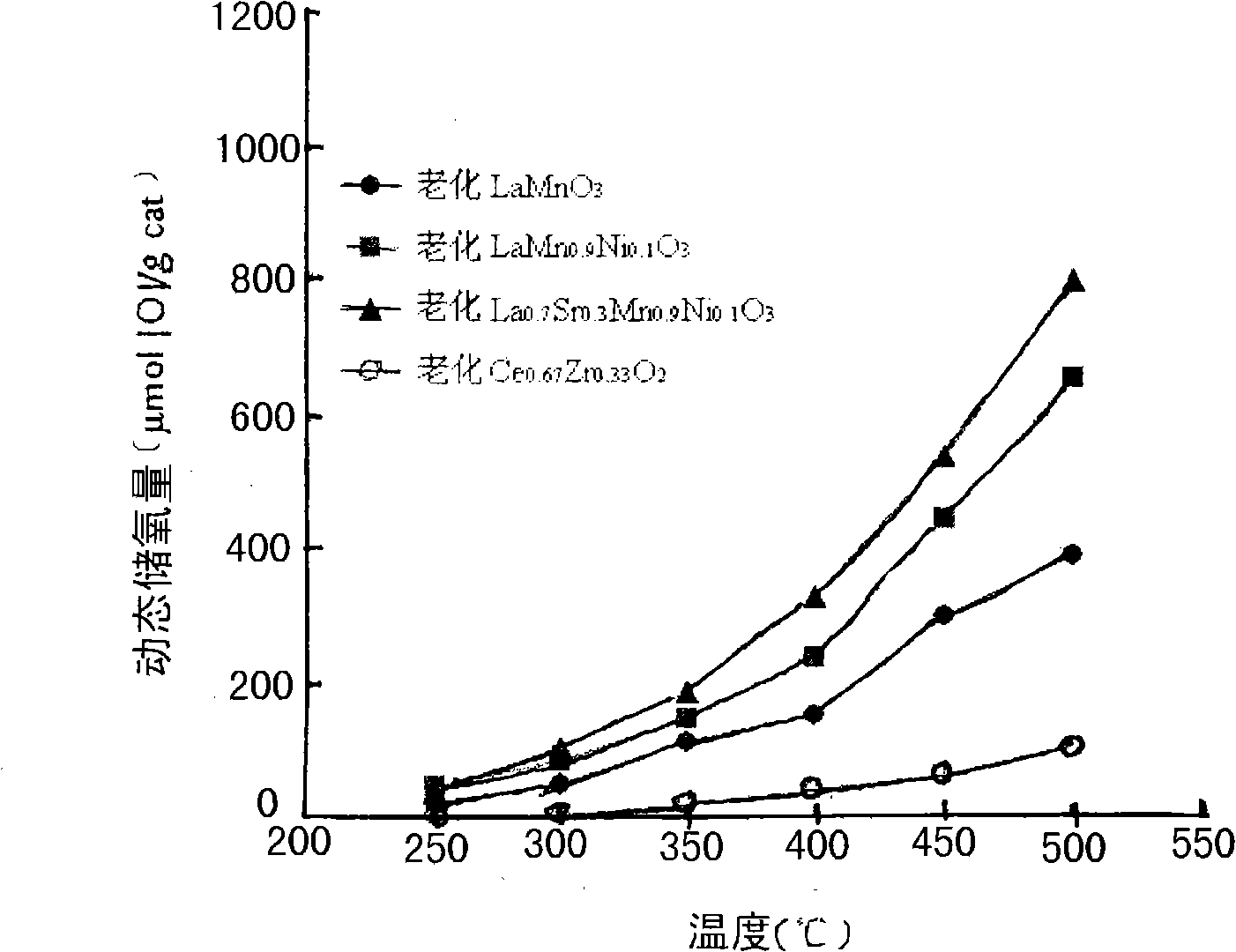
Rare earth perovskite type oxygen storage material for purifying vehicle tail gas
An oxygen storage material, a technology for automobile exhaust, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as poor low-temperature oxygen storage performance, and achieve excellent Medium and low temperature oxygen storage performance and high temperature thermal stability, simple process, and the effect of widening the operating window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Lanthanum nitrate [La(NO 3 ) 3 ·6H 2 O] 6.93g, manganese nitrate [50wt%Mn(NO 3 ) 2 Solution] 5.73g was placed in 40ml deionized water, stirred and dissolved, and 6.72g citric acid was added under room temperature and stirring conditions, and after dissolving, 0.67g ethylene glycol was added dropwise. After the dropwise addition, heated and stirred at 80°C until Evaporation of water produces viscous colloid. Dry the obtained colloid in air atmosphere at 110°C for 12 hours, take it out and pulverize it, pre-calcine at 300°C for 1 hour, calcinate at 800°C for 3 hours, and cool in the furnace to obtain a black powder.
[0013] identified by X-ray diffraction (XRD) analysis (e.g. figure 1 Shown), the powder is made of LaMnO 3 A single crystal composed of a perovskite-structured composite oxide.
Embodiment 2
[0015] Lanthanum acetate [La(CH 3 COO) 3 2H 2 O] 5.63g, manganese acetate [Mn(CH 3 COO) 2 ] 2.49g, nickel acetate [Ni(CH 3 COO) 2 ·H 2 O] 0.31g was placed in 40mL deionized water, stirred until fully dissolved, then heated and stirred at 80°C to make it fully hydrolyzed until the water evaporated to produce a viscous colloid. Dry the obtained colloid at 110°C for 12 hours in an air atmosphere, take it out, grind it finely, and calcinate it. The calcination process was performed in the same manner as in Example 1 to obtain a black powder.
[0016] Identified by X-ray diffraction analysis results (such as figure 1 Shown), the powder is made of LaMn 0.9 Ni 0.1 o 3 A single crystal composed of a perovskite-structured composite oxide.
Embodiment 3
[0018] Lanthanum chloride [LaCl 3 ·7H 2 O]4.16g, strontium nitrate [Sr(NO 3 ) 2 ] 1.02g, manganese acetate [Mn(CH 3 COO) 2 ] 2.49g, nickel nitrate [Ni(NO 3 ) 2 ·5H 2O] 0.44g is placed in 40mL deionized water, after stirring and dissolving, the mixed solution is slowly dropped into excess ammonia solution (pH=9~11), and at the same time, the ammonia solution is stirred rapidly to make the precipitation sufficient and uniform, and it is allowed to stand after dropping After 6-12 hours, filter, and dry the obtained precipitate in air atmosphere at 110°C for 12 hours, take it out, grind it finely, and calcinate. The calcination process was performed in the same manner as in Example 1 to obtain a black powder.
[0019] Identified by X-ray diffraction analysis results (such as figure 1 Shown), the powder is made of La 0.7 Sr 0.3 mn 0.9 Ni 0.1 o 3 A single crystal composed of a perovskite-structured composite oxide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com