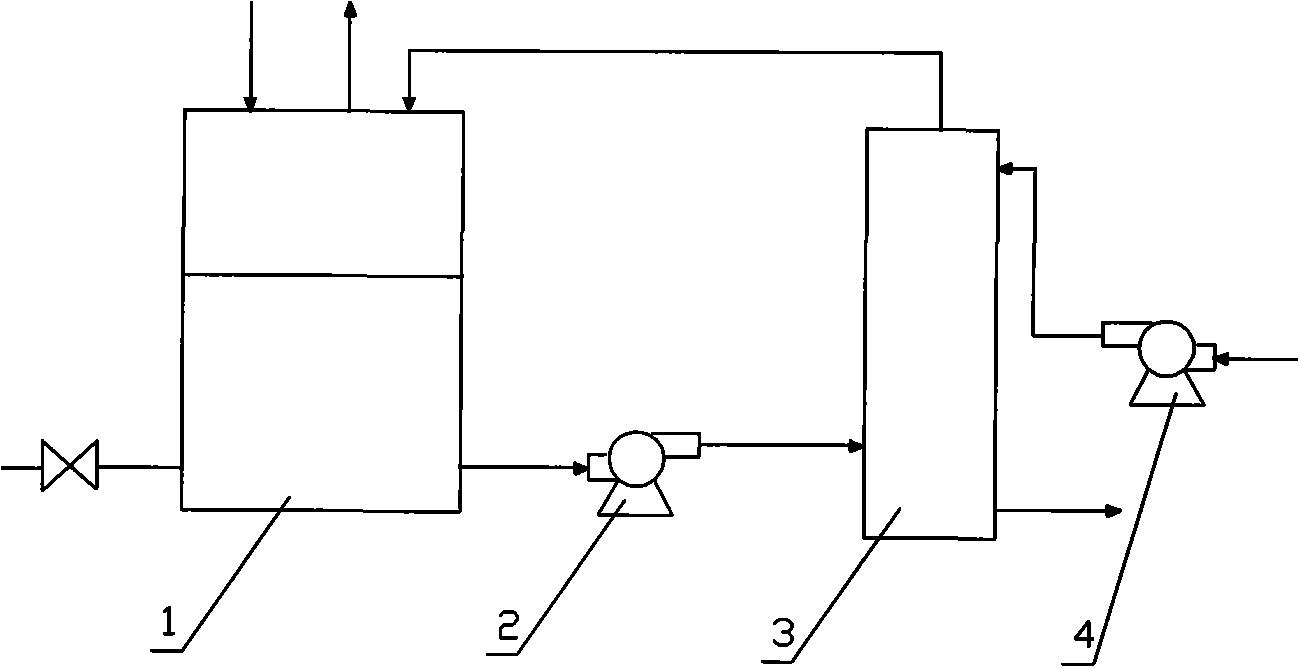
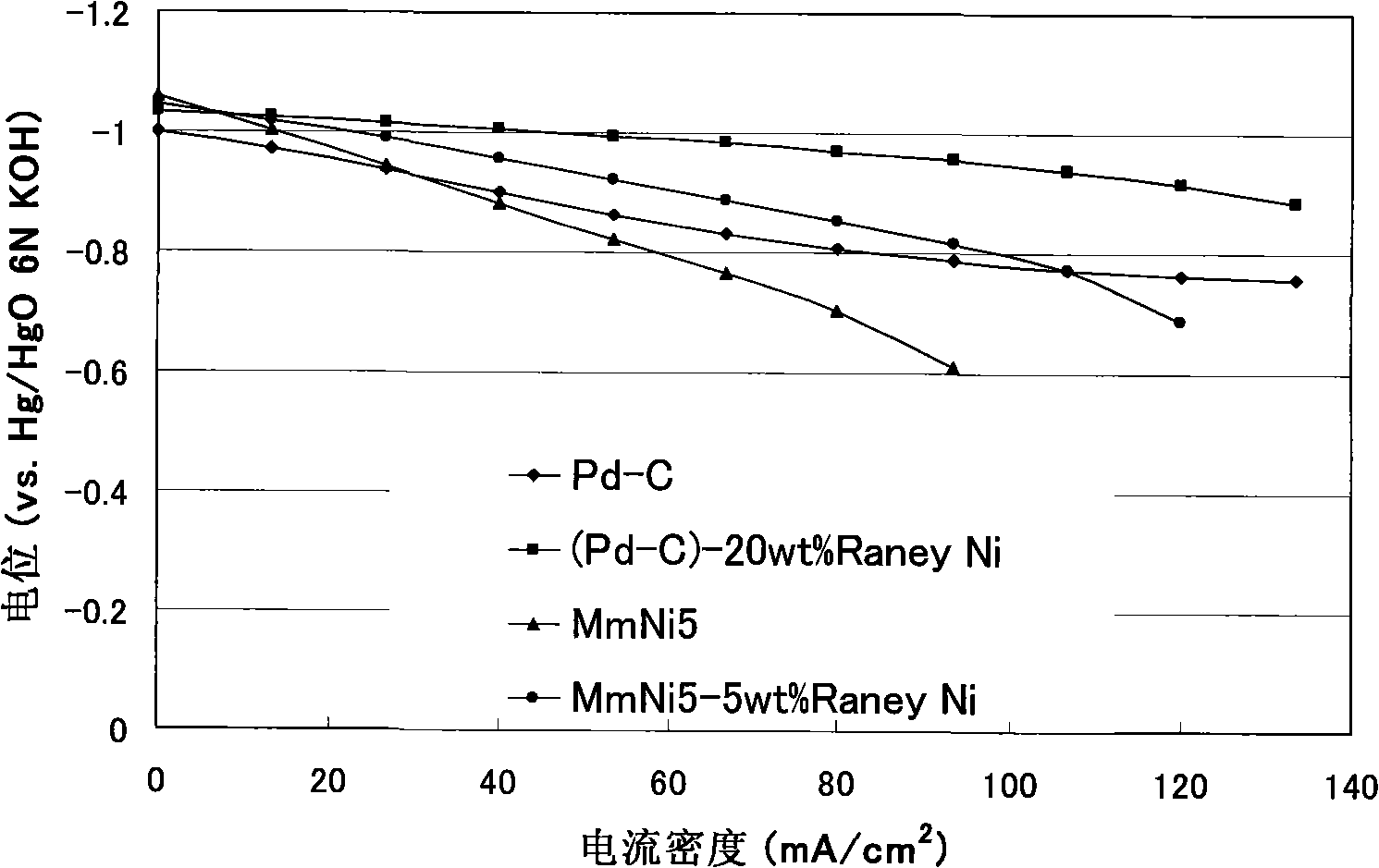
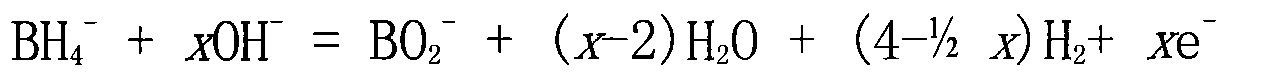Direct sodium borohydride-hydrazine mixed fuel cell
A sodium borohydride, fuel cell technology, applied in indirect fuel cells, fuel cells, fuel cell components and other directions, can solve the problems of no application, poor reaction activity, poor performance, etc., to improve stability and increase production. Hydrogen, the effect of improving work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the modulation of sodium borohydride-hydrazine mixed hydrogen generation solution
[0052] At room temperature, dissolve 100g of sodium borohydride in 500ml of water and hydrazine (N 2 h 4 ·H 2 0), the granular sodium hydroxide of 50g is dissolved in 400ml distilled water, after stirring at room temperature, obtain sodium borohydride-hydrazine alkaline mixed fuel.
Embodiment 2
[0053] Embodiment 2: the preparation of direct fuel cell (1)
[0054] A direct fuel cell contains an anode, an anion exchange membrane, and a cathode. The cathode is a purchased platinum carbon electrode, and the coating amount of platinum is 1mg Pt / cm 2 , the anode is the purchased palladium carbon electrode, and the coating amount of palladium is 1mgPd / cm 2 . The catalyst is coated on carbon fiber paper, and the side of the electrode coated with the catalyst is facing the anion exchange membrane, at 100kg / cm 2 , Hot pressing at 150 degrees to make a membrane electrode, and assemble a direct fuel cell with a plate engraved with a flow path.
Embodiment 3
[0055] Embodiment 3: the preparation of direct fuel cell (2)
[0056] A direct fuel cell contains an anode, an anion exchange membrane, and a cathode. The cathode is a platinum carbon electrode, and the coating amount of platinum is 1mg Pt / cm 2 , the anode catalyst is a metal hydride (MmNi 5 ). MmNi 5 The preparation method of the catalyst is: weigh 20g of hydrogen storage material, put it into a ball mill tank, which contains 7 stainless steel balls with a diameter of 1 cm, then add the 0.2M aqueous solution of the precursor of the above catalyst, tighten the cover, and produce it through a high-speed planetary mill. With an acceleration of 1G, ball milling at room temperature for 90 minutes, rinsing with distilled water for 5 times, and vacuum drying below 80°C for 3 hours, MmNi was obtained. 5 Catalyst 18g. Add 54ml of distilled water, 54ml of ethanol, 126ml of perfluoropolysulfonic acid resin of 5wt%, 9ml of glycerin, and mix uniformly with ultrasonic waves to form a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com